Draw the structure and name the product formed if the following alcohols are oxidized. Assume that an excess of oxidizing agent is used.
(i) CH3CH2CH2CH2OH
(i) 2-butenol
(iii) 2-methyl-1-propanolSolution in Kannada
(i) CH3CH2CH2COOHButanoic acid
(ii) CH3−CH=CH−COOHBut-2-en-1-oic acid or 2-butenoic acid
(iii) CH3−CH(CH3)−COOH2-methyl propanoic acid
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OSWAAL PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - TOPIC -2 METHODS OF PREPARATION OF ALKANOLS (LONG ANSWER TYPE QUESTIONS -I)
2 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OSWAAL PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - TOPIC -2 METHODS OF PREPARATION OF ALKANOLS (LONG ANSWER TYPE QUESTIONS -II)
1 Video
- ALCOHOLS, PHENOLS AND ETHERSBOOK - OSWAAL PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - TOPIC -2 METHODS OF PREPARATION OF ALKANOLS (VERY SHORT ANSWER TYPE QUESTIONS)
5 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBOOK - OSWAAL PUBLICATIONCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDSEXERCISE - TOPIC -3 METHODS OF PREPARATION OF CARBOXYLIC ACID, PROPERTIES AND USES (LONG ANSWER TYPE QUESTIONS II)
7 Videos
Similar Questions
What is lucas reagent? How does it help in distinguishing primary, secondary and tertiary alcohols?
View SolutionExplain the preparation of phenol from cumene.
View SolutionHow would you obtain ethan-1,2-diol from ethanol?
View SolutionA ketone A(C4H8O) which undergoes a haloform reaction gives compound B on reduction. B on heating with sulphuric acid gives a compound C whch forms mono-ozonide D. D on hydrolysis in presence of zinc dust gives only acetaldehyde E. Identify A,B,C,D and E. Write the reaction involved.
View SolutionThe primary alcohol 1 - Propanol can be oxidized with Aqueous nitric acid HNO3 are required for complete oxidation of 180 gm of 1-Propanol ?
CH3CH2OH+HNO3→CH3CH2COOH+NO+H2O
View SolutionHydroboration-Oxidation of CH3CH=CH2 produces
(1). CH3CH2CH2OH
(2). CH3CH(OH)CH2
(3). CH3CH(OH)CH2OH
(4). CH3COCH3
View SolutionClassify the following as primary, secondary and tertiary alcohols: (i) CH_(3)-overset(CH_(3))overset("| ")underset(CH_(3))underset("| ")(C )-CH_(2)OH (ii) H_(2)C=CH-CH_(2)OH (iii) CH_(3)-CH_(2)-CH_(2)-OH (iv)
View SolutionDraw the structrue and name the product formed if the following alcohols are oxidized. Assume that an excess of oxidising agent is used.
(i) CH3CH2CH2CH2OH
(ii) 2-butenol
(iii) 2-methyl-1-propanol
View SolutionDraw the structure and name the product formed if the following alcohols are oxidised. Assume than an excess of oxidising agent is used. (i) CH_(3)CH_(2)CH_(2)CH_(2)OH (ii) 2-butenol (iii) 2-methyl-1-propanol.
View Solutionनिम्न ऐल्कोहॉल को जल में विलेयता के बढ़ते क्रम में व्यवस्थित कीजिये -
(i) CH3OH (ii) CH3CH2OH
(iii) CH2OH∣CH2OH (iv) CH2OH∣CHOH∣CH2OH
View SolutionAn alcohol on oxidation is found to give CH_(3)COOH and CH_(3)CH(OH)CH_(2)CH_(2)CH_(3) The structure of the alcohol is :
View SolutionClassify the following as primary, secondary and tertiary alcohols:
(i) CH3−CH3| C| CH3−CH2OH (ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
(iv)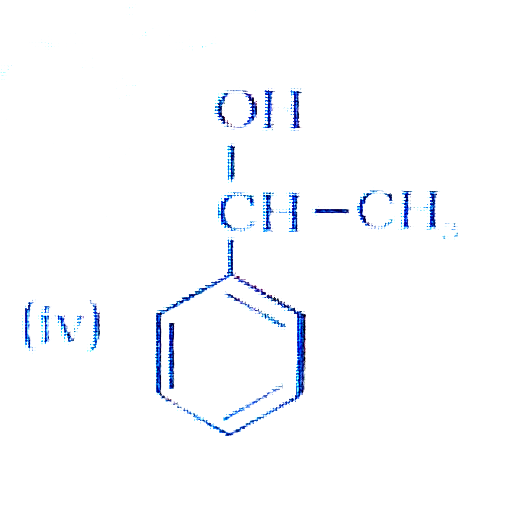
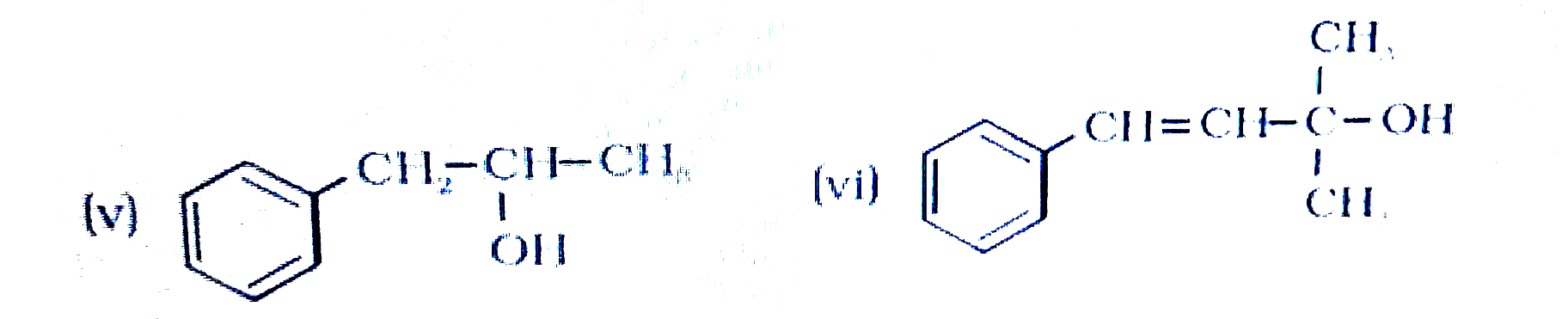
View SolutionClassify the following as primary alcohols, secondary alcohols and tertiary alcohols.
(i) CH3−CH3∣C∣CH3−CH2OH
(ii) H2C=CH−CH2OH
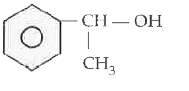
(iv) CH3−CH2−CH2−OH
View SolutionDraw the structrue and name the product formed if the following alcohols are oxidized. Assume that an excess of oxidising agent is used. (i) CH_(3)CH_(2)CH_(2)CH_(2)OH (ii) 2-butenol (iii) 2-methyl-1-propanol
View SolutionClassify the following as primary, secondary and tertiary alcohols :
(i) CH3−CH3| C | CH3−CH2OH
(ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
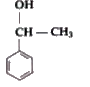

View Solution

