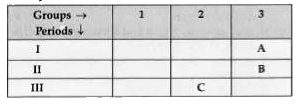
The position of three elements A, B and C in the periodic table are shown below :

Give reasons to explain the following:
(i) Element 'A' is a metal.
(ii) Element 'B' has larger size than 'C'.
(iii) Element 'C' has a valency - 3. Solution in Kannada
(i) Element .A. is a metal because it is present in group I (1 valence electron) and can lose electron easily.
(ii) Element B has larger size than .C. because it has more number of shells than .C..
Also, B lies in the third period and has three shells whereas B lies in second period and has two shells.
(iii) .C. being an element of 3rd group has three valence electrons, therefore its valency is 3.
Similar Questions
The position of three elements A, B and C in the periodic Table is shown below: (a) State whether A is a metal or non-metal (b) State whether C is more reactive or less reactive than A ( c) Will C be larger or smaller in size than B? (d) Which type of ion, cation or anion, will be formed by element C?
View SolutionThe position of three elements A, B and C in the periodic table are as shown below:
 State whether a is a metal or non-metal
State whether a is a metal or non-metal
View SolutionThe position of three elements A, B and C in the periodic table are as shown below: Will C be larger or smaller in size than B
View SolutionThe position of three elements A, B and C in the periodic table are as shown below: Which type ion, cation or anion, will be formed by elements A ?
View SolutionThe position of three elements A, B and C in the periodic table is as follows. Now tell me Will C be larger or smaller than B?
View SolutionThe position of elements A,B,C,D and E in the periodic table are shown below :

Which is larger in size (i) D or E (ii) B or C.
View SolutionThe positions of three elements A, B and C in the periodic table are shown below:
Group 16Group 17−−−A−−BC
Will C be larger or smaller in size than B?
View Solution
