The position of elements A, B, C, D in the modern periodic table is given in the following table. Answer the following questions by observing the table:
Group 1Group 2Period 3ABPeriod 4CD
i] Which element has the highest atomic size? Why?
ii] Which element has the least metallic property? Why? Solution in Kannada
i] C has highest atomic size since atomic size increases down a group and decreases along a period from left to right.
ii] B has least metallic property since metallic property increases down a group and decreases along a period.
Similar Questions
Elements in the modern periodic table are arranged in ………… groups and ……………. Periods.
View Solution(i) आधुनिक आवर्त सारणी में कितने आवर्त और कितने समूह ( वर्ग ) है ?
(ii) आवर्त सारणी के प्रथम आवर्त में केवल दो ही तत्व क्यों है ?
View SolutionThe atomic number of elements A, B, C, D and E are given below:

From the above table, answer the following questions :
(i) Which two elements are chemically similar ?
(ii) Which is an inert gas ?
(iii) Which element belongs to 3 period of periodic table ?
(iv) Which element among these is a non-metal ?
View SolutionGroup I elements in the Modern Periodic Table are known as
View Solutiona] Write the trend of atomic size and metallic character along a group and a period in the Modern Periodic Table.
b]"Elements in the periodic table show periodicity of properties." List any four such properties.
View SolutionThe following tables shows the position of six elements A, B, C, D, E and F in the periodic table? Using the table answer the following question: which element is non- metal with valency of 3?
View SolutionThe following tables shows the position of six elements A, B, C, D, E ,and F in the periodic table? Using the table answer the following question:Out of D and E, which one has a bigger atmic radius and why?
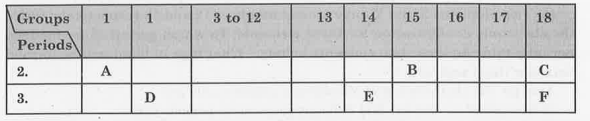
View Solution