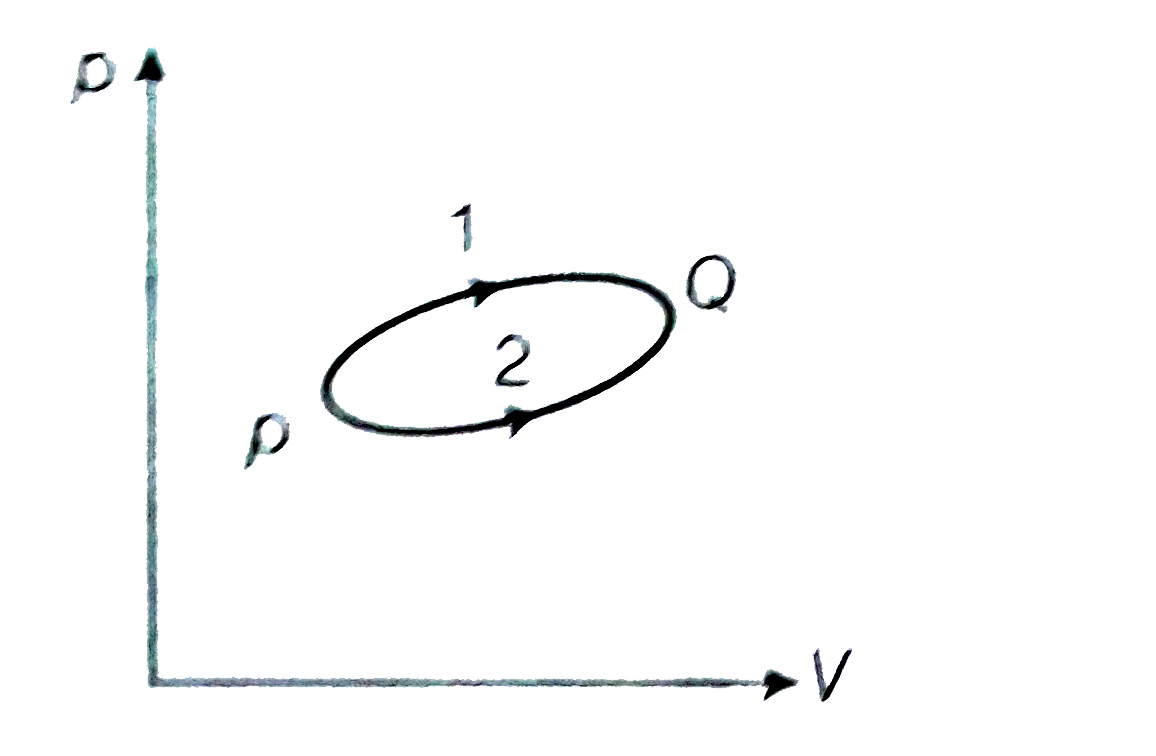
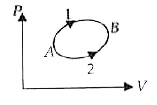
A system goes from P to Q by two different paths in the P -V diagram as shown in figure. Heat given to the system in path 1 is 1000 J. The work done by the system along path 1 is more than path 2 by 100 J. What is the heat exchanged by the system in path 2 ?
 Solution in Gujrati
Solution in Gujrati
For path 1: Heat given Q1=+1000 J
Work done = W1
For path 2: Work done W2=W1−100J
Heat given Q2= ?
As change in internal energy between two states for different path is same.
∴ΔU=Q1−W1=Q2−W2
∴1000−W1=Q2−(W1−100)
∴Q2=1000−W1+W1−100
∴Q2=900 J
Similar Questions
When a system is taken from stata a to state b along the path acb as shown in figure, 60 J of heat flows into the system and 30 J of work is done by the system. Along the path adb, if the work done by the system is 10 J, heat flow into the system is
View SolutionA system goes from P to Q by two different paths in the P-V diagram as shown in figure. Heat given to the system in path 1 is 1100 J, the work done by the system along path 1 is more than path 2 by 150 J. The heat exchanged by the system in path 2 is
View SolutionA system goes from P to Q by two different paths in the p−V diagram as shown in figure. Heat given to the system in path 1 is 1000 J. The work done by the system along path 1 is more than path 2 by 100 J. What is the heat exchanged by the system in path 2 ?
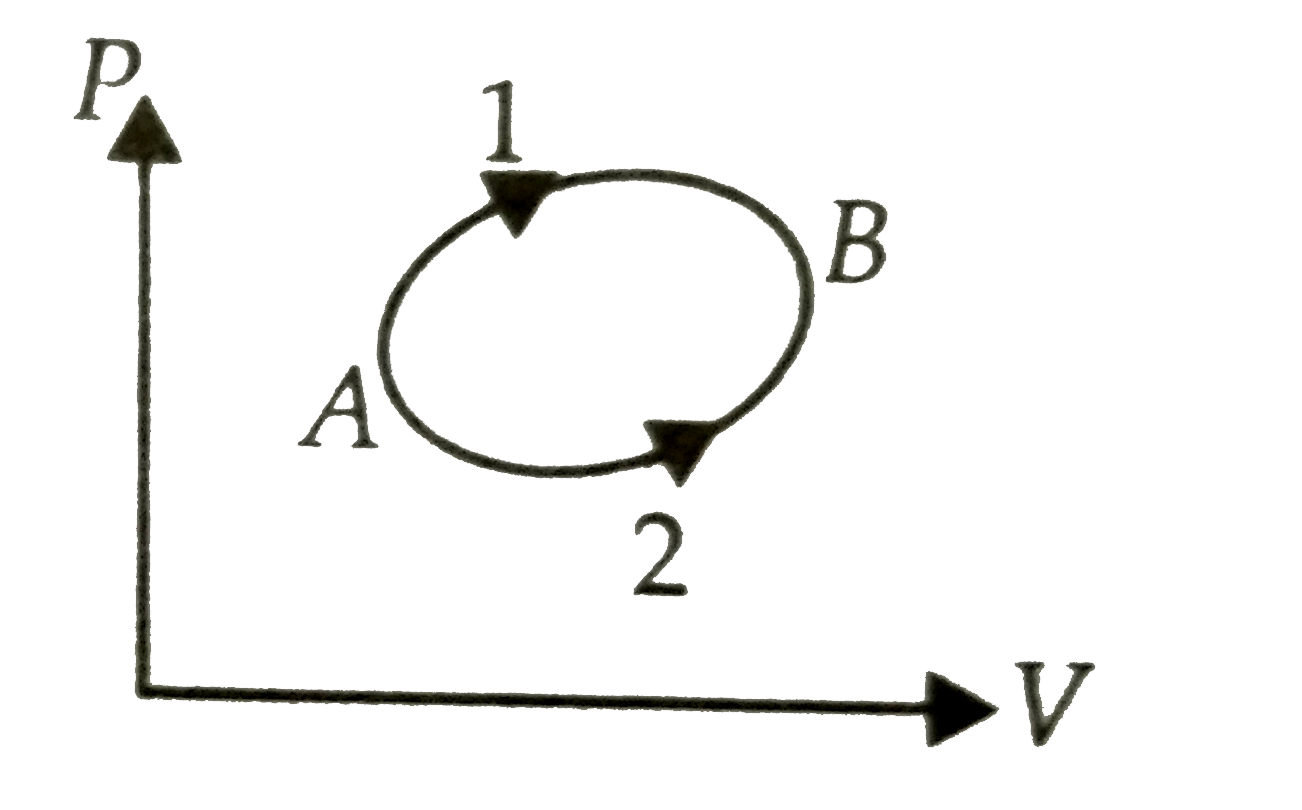
View SolutionA gas can be ken from A to B via two different processes ACB and ADB. When path ACB is used 60 J of heat flows into the system and 30 J of work is done by the system. If path ADB is used work done by the system is 10 J. The heat Flow into the system in path ADB is :
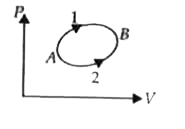
View SolutionA system goes from A to B by two different paths in the P-V diagram as shown in figure Heat given to the system in path 1 is 1100 j, the work done by the system along path 1 is more than path 2 by 150 J. The heat exchanged by the system in path 2 is
View Solutionदर्शाए P-V आरेख में कोई निकाय P से Q तक दो विभिन्न पथों द्वारा जाता है। पथ 1 पर निकाय को दी गई ऊष्मा 1000 J है। निकाय द्वारा पथ 1 के अनुदिश किया गया कार्य पथ 2 की तुलना में 100 J अधिक है। पथ 2 में निकाय द्वारा ऊष्मा विनिमय क्या है?
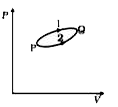
View Solutionएक निकाय चित्र में दर्शाए अनुसार P- V ग्राफ में दो विभिन्न पथों द्वारा A से B तक जाता है। पथ । में निकाय को दी गई ऊष्मा 1100 Jहै, पथ । के अनुदिश निकाय द्वारा किया गया कार्य पथ 2 से 150 J अधिक है। पथ 2 में निकाय द्वारा ऊष्मा विनिमय है
View Solution

