Heat absorbed by the gas undergo a cylic process along closed path in one complete cycle as shown in figure will be _____ J.
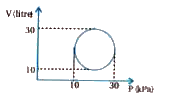 Solution in Gujrati
Solution in Gujrati
107π
104π
102π
10−3π
The correct Answer is:C
For cyclic process ΔU=0
Heat change for a system ΔQ=ΔU+ΔW
ΔQ=ΔU+Δ(PV)
∴ΔQ = Area under the graph,
=πr2kPa×lit
=π(30−102)2 kPa x lit
=π(202)∘kPa×lit
=π×100×103×10−3 J
=100πJ=102πJ
Similar Questions
एक आदर्श गैस को, चित्र में दर्शाये गये अनुसार चक्रीय प्रक्रम abca से गुजारा जाता है। ca पथ के अनुदिश गैस की आन्तरिक ऊर्जा में परिवर्तन -180 J है। ab पथ के अनुदिश, गैस 250 J ऊष्मा अवशोषित करती है तथा bc पथ के अनुदिश, गैस 60 J ऊष्मा अवशोषित करती है तो. पथ abc के अनुदिश, गैस द्वारा किया गया कार्य है
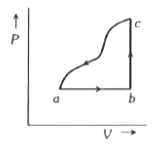
View SolutionA sample of an ideal gas is taken through the cyclic process abca and shown in the figure. The change in the internal energy of the gas along the path ca is -180J . The gas absorbs 250J of heat along the path ab and 60 J along the path bc. the work done by the gas along the path abc is:
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure. Calculate
The net heat absorbed by the gas in the path BC.
View Solutionचित्र में दर्शाए गए अनुसार चक्रीय प्रक्रम से गुजरने वाले निकाय के द्वारा अवशोषित ऊष्मा है-
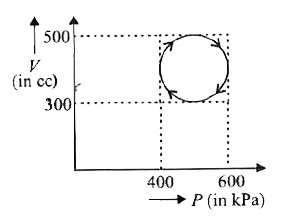
View SolutionOne mole of a gas is taken through a cycle as shown in figure calculate. the amount of heat absorb in AB path.
View SolutionOne mole of a gas is taken through a cycle as shown in figure calculate.
Total amount of heat absorb in BC path.
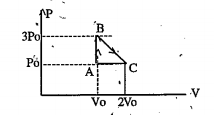
View SolutionOne mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in figure . Calculate the net heat absorbed by the gas in the path BC.
View SolutionAn ideal gas follows a cyclic process as shown in figure. Internal energy of gas at point A is 10 J while at B is 70 J. the heat absorbed by gas in BC is 120 J.
(a) How much heat is absorbed in process AB?
(b) Find heat energy rejected or absorbed by gas in process CA.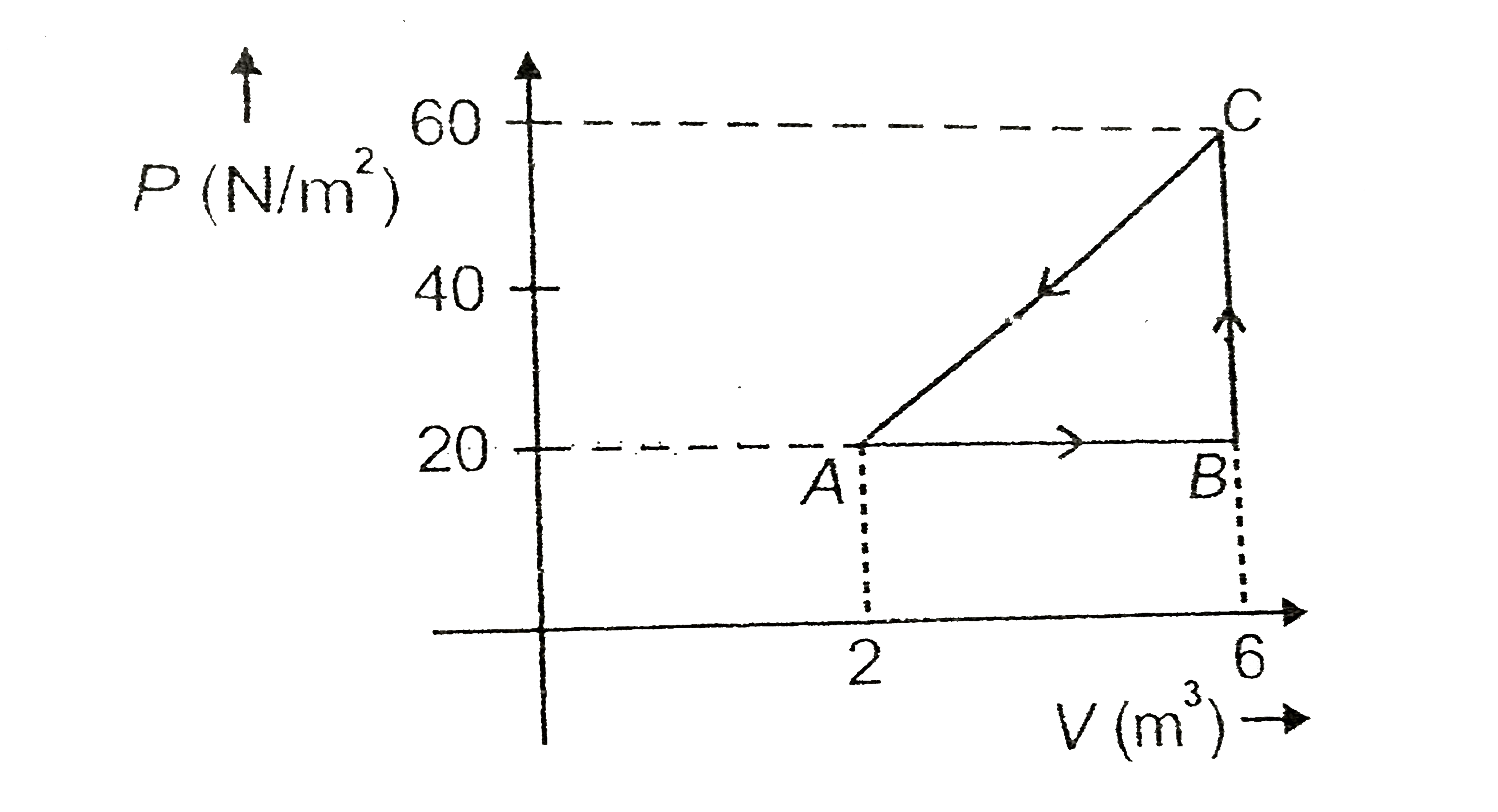
View Solution
