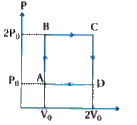
Helium gas goes through a cycle ABCDA as shown in figure. Efficiency of this cycle is nearly _______ (Imagine He is an ideal gas)
 Solution in Gujrati
Solution in Gujrati
9.1%
10.5%
12.5%
15.4%
The correct Answer is:D
Work done in complete cyclic = Area under P-V graph
(Cyclic process is clockwise so work is positive)
∴ W=Area of ABCD
=AB x BC
=(2P0−P0)×(2V0−V0)
∴W=P0V0 …(1)
For He gas,
Specific heat at constant volume Cv=32R and
Specific heat at constant pressure Cp=52R
Heat absorbed (along path AB) at constant volume ,
(ΔQ)AB=μCVΔT=32μRΔT
=32×ΔPV0[∵PV=μRT]
=32(2P0−P0)V0=32P0V0
Heat absorbed (along path BC ) at constant pressure,
(ΔQ)BC=μCPΔT=52μRΔT
=52(2P0)ΔV[∵μRΔT=PV]
=52(2P0)(2V0−V0)
=52(2P0×V0)
=5P0V0
Heat is not absorbed at path CD and DA,
∴ Total heat absorbed Q1=32P0V0+5P0V0
=132P0V0 ...(2)
∴ Efficiency η=WQ1
=P0V0132P0V0 (∵ From eq.(1) and (2))
=213×100%
=15.38%
≈ 15.4%
Similar Questions
Helium gas goes through a cycle ABCDA (consisting of two isochoric and isobaric lines) as shown in figure Efficiency of this cycle is nearly: (Assume the gas to be close to ideal gas)
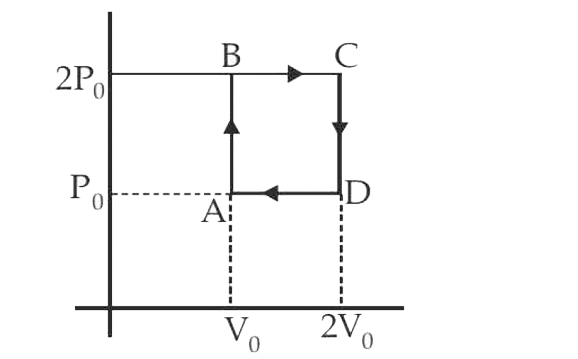
View Solutionहीलियम गैस एक चक्र ABCDA से गुजरती है। ABCDA दो समआयतन और दो समदाब रेखाओ से बना है जैसा कि चित्र में दर्शाया गया है। इस चक्र कि दक्षता है (यह मान ले कि गैस लगभग आदर्श गैस है)
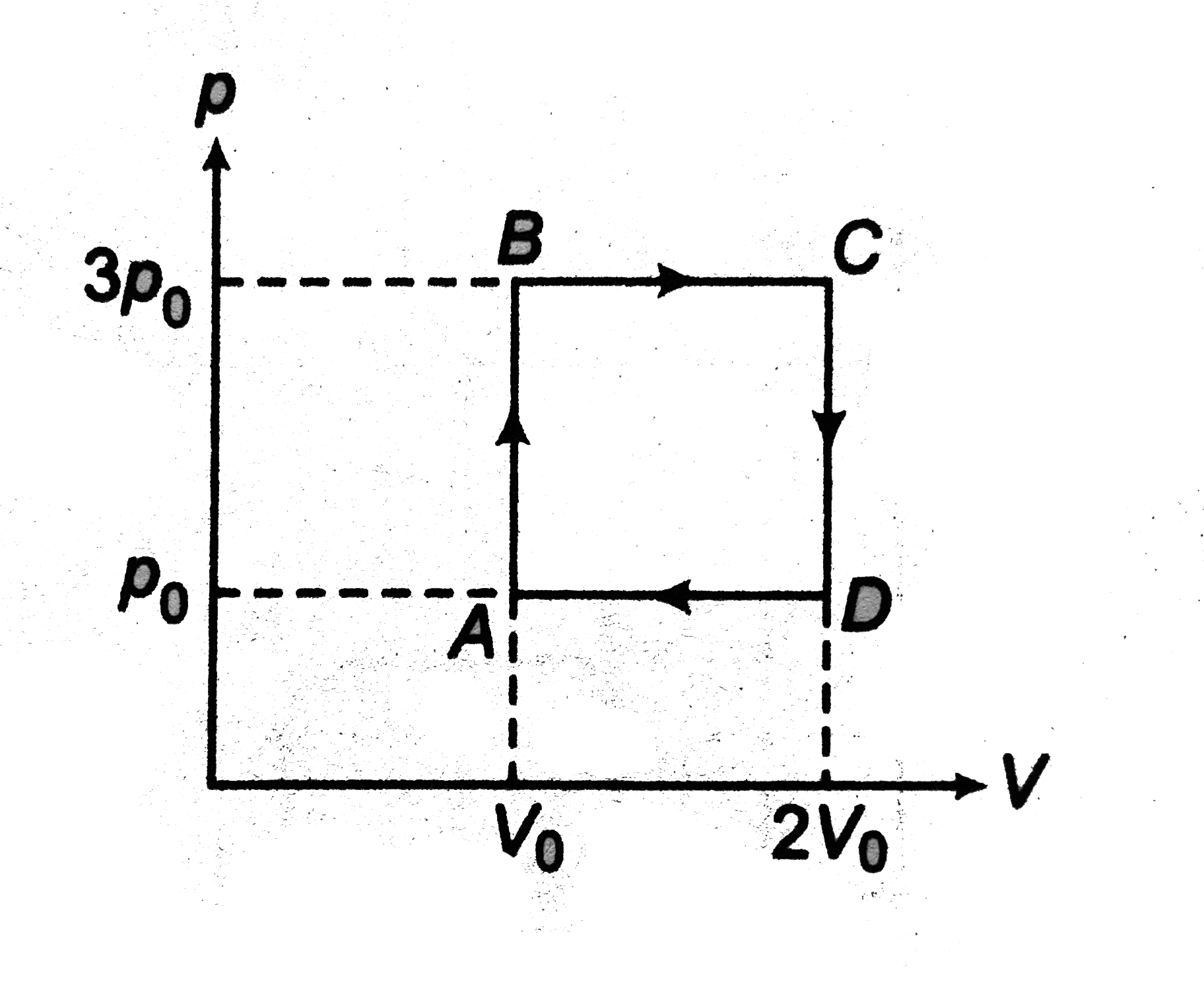
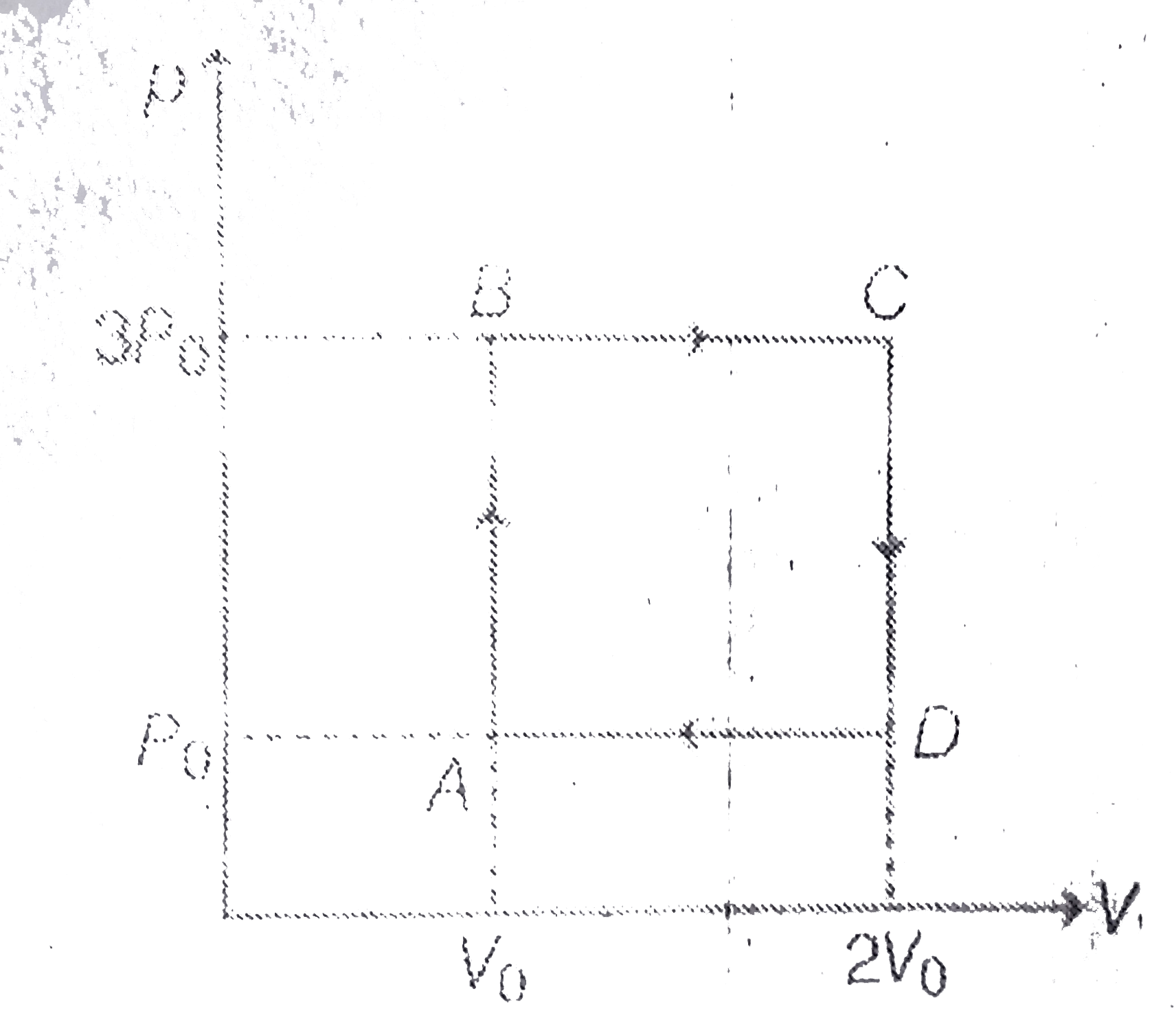
View SolutionAn ideal monoatomic gas is carried around the cycle ABCDA as shown in the figure. The efficiency of the gas cycle is
View SolutionTwo moles of a monatomic ideal gas undergo a cyclic process ABCDA as shown in figure. BCD is a semicircle. Find the efficiency of the cycle.
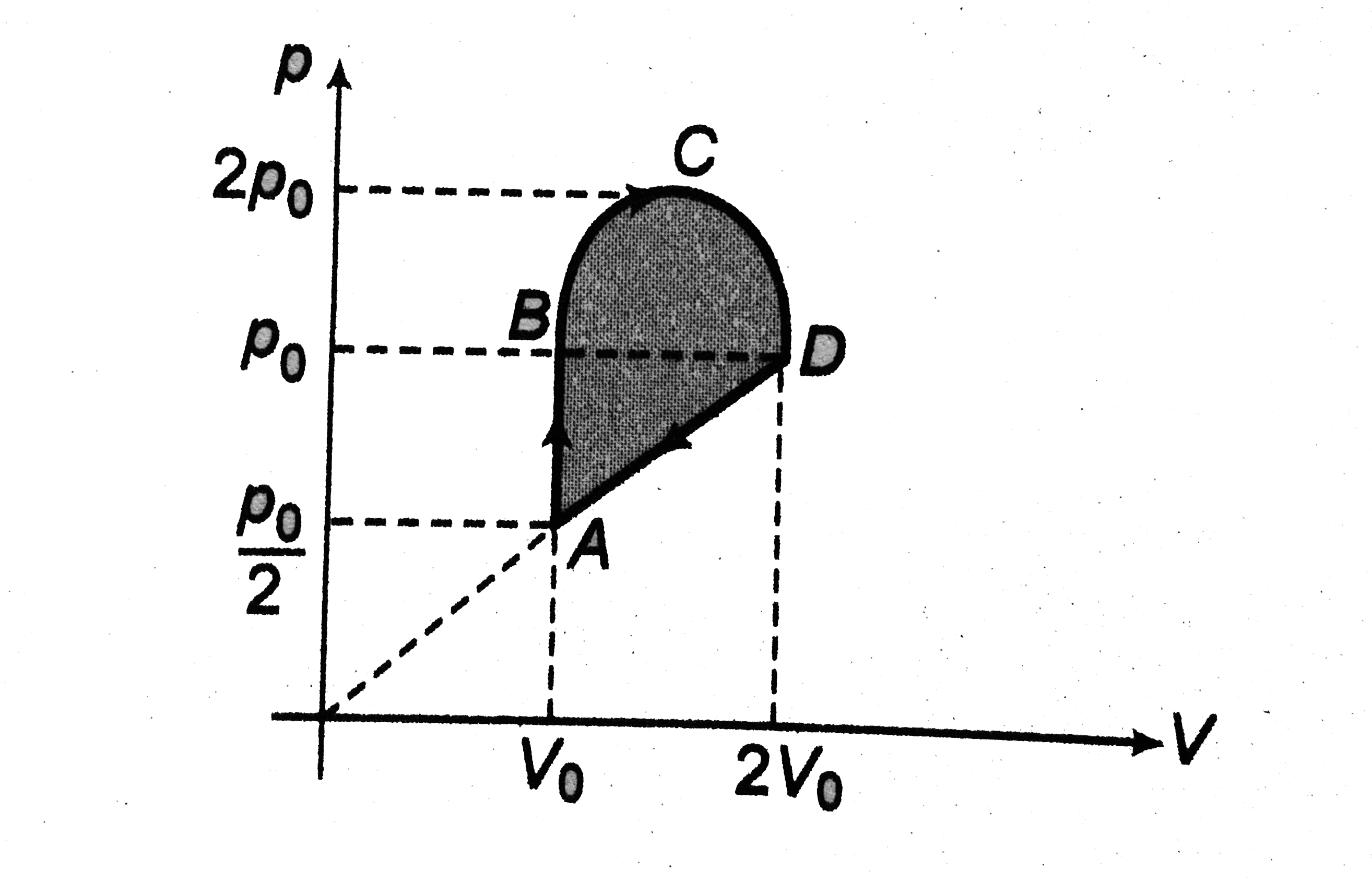
View SolutionAn ideal monoatomic gas is carried around the cycle ABCDA as shown in the figure. The efficiency of the cycle is ltBrgt
View SolutionTwo moles of helium gas are taken over the cycle ABCDA, as shown in the P-T diagram
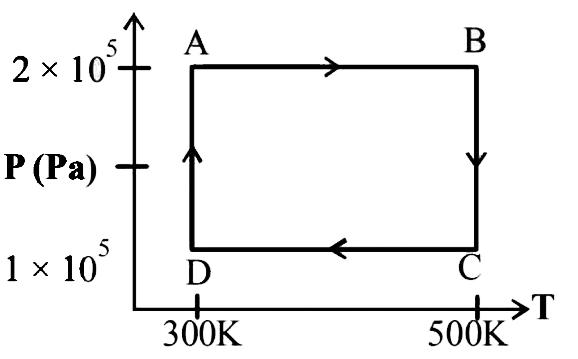
The net work done on the gas in the cycle ABCDA is :
View SolutionHelium gas goes through a cycle ABCDA (consisting of two isochoric and isobaric lines) as shown in figure Efficiency of this cycle is nearly: (Assume the gas to be close to ideal gas)

View Solution
