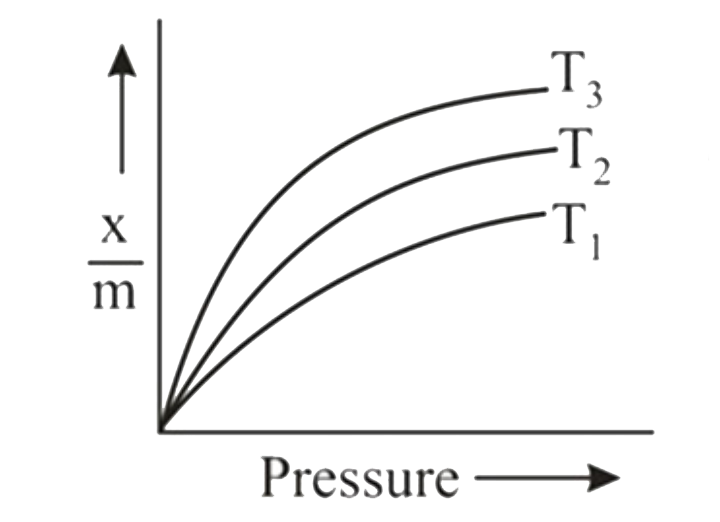
The following figure shows the variation of adsorption of N, on charcoal with pressure at different constant temperatures.

Arrange the temperatures in increasing order.
Temperatures in the increasing order are
T1<T2<T3
Topper's Solved these Questions
- SURFACE CHEMISTRYBOOK - U-LIKE SERIESCHAPTER - SURFACE CHEMISTRYEXERCISE - SHORT ANSWER QUESTIONS
39 Videos
- SURFACE CHEMISTRYBOOK - U-LIKE SERIESCHAPTER - SURFACE CHEMISTRYEXERCISE - LONG ANSWER QUESTIONS-I
76 Videos
- SURFACE CHEMISTRYBOOK - U-LIKE SERIESCHAPTER - SURFACE CHEMISTRYEXERCISE - FILL IN THE BLANKS
8 Videos
- SOLUTIONBOOK - U-LIKE SERIESCHAPTER - SOLUTIONEXERCISE - SELF ASSESSMENT TEST (SECTION D)
2 Videos
- THE D-AND F-BLOCK ELEMENTS BOOK - U-LIKE SERIESCHAPTER - THE D-AND F-BLOCK ELEMENTS EXERCISE - SELF ASSESSMENT TEST (SECTION A ) MULTIPLE CHOICE QUESTIONS (CHOOSE THE CORRECT OPTION)
7 Videos
Similar Questions
Adsorption of a gas on the surface of solid is generally accompanied by decrease in entropy. Still it is a spontaneous process. Explain.
View SolutionWhat happens when
a freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution ?
View SolutionUnder very high vaccum of the order of __________, it is now possible to obtain ultra clean surface of the metals.
View Solution(a) 30 g of urea (M = 60 g mol−1) is dissolved in 846 g of water. Calculate the vapour pressure of water for this solution if vapour pressure of pure water at 298 K is 23.8 mmHg. (b) Write two differences between ideal solutions and non-ideal solutions.
View SolutionWhich of the following is not a transition element ?
View SolutionConsider the adsorption isotherm given below and interpret the variation in the extent of adsorption (x/m) when a. Temperature increased at constant pressure. b. Pressure increases at constant temperature.
View SolutionA cusrve showing the variation of extent of adsorption with temperature at constant pressure is called……………
View SolutionFollowing is the variation of physical adsorption with temperature.
View SolutionThe correct ascending order of adsorption of the following gases on the same mass of charcoal at the same temperature and pressure is
View SolutionN2,CO एवं CH4 के क्रांतिक ताप क्रमशः 126, 134 एवं 190K है। इनको सक्रियित चारकोल पर अधिशोषण के बढ़ते क्रम में व्यवस्थित कीजिए।
View SolutionThe variation of extent of adsorption with pressure at a given constant temperature is given in following figure
Which of the following relation between temperature of isotherms is correct ?
View SolutionThe curve showing the variation of adsorption with pressure at constant temperature is called:
View SolutionThe correct ascending order of adsorption of the following gases on the same mass of charcoal at the same temperature and pressure is
View SolutionThe curve showing the variation of pressure with temperature for a given amount of adsorption is called
View SolutionThe curve showing the variation of adsorption with pressure at constant temperature is is called :
View SolutionThe curve showing the variation of adsorption with pressure at constant temperature is is called :
View Solution

