A gas is produced when conc. H2SO4 is added to solid sodium chloride taken in a test tube. The gas coming out of the delivery tube is passed over a dry blue litmus paper and then over a moist blue litmus paper. What would you observe ? Explain reason with the help of a chemical equation.
HCl gas is produced when conc. H2SO4 is added to solid sodium chloride.
NaCl+H2SO4→NaHSO4+HCl
HCl gas when passed over dry blue litmus paper shows no effect.
HCl gas when passed over moist blue litmus paper turns it red.
This is because dry HCl gas does not produce H+ ions, therefore there is no action on dry blue litmus paper. Moist blue litmus paper contains water. HCl gas when passed over moist blue litmus paper reacts with water to form H+ ions. An acid (containing H+ ions) turns blue litmus red. Therefore, a red colour will be obtained.
Similar Questions
(a) A gas is produced when conc. H2SO4 is added to solide sodium chloride taken in a test tube and the tube is heated. The gas coming out through the delivery tube is passed over a dry litmus paper and then over a moist litmus paper. What would you observe ? Explain your answer.
(b) Fresh mikl has pH of 6. When it changes to curd (yogurt(, will its pH value increase or decrease? Why ?
(c ) What will be the colour of blure limus in a solution of sodium carbonate ?
View SolutionWhat do you observe when dil. HCl is added on Zn pieces taken in a test tube? How would you identify the gas evolved? Write on use of this gas.
View Solution
Knowledge Check
Sodium chloride was taken in a test tube and conc. H2SO4 acid added to it and the gas was tested with a moist litmus paper. Give your observation.
AThe paper got bleached.BThe paper caught fireqCNothing happened to the paper.DThe paper turned red.A student prepared hydrogen chloride gas by treating sodium chloride with concentrated sulphuric acid in a test tube. He held a strip of dry blue litmus paper in HCl gas coming out the test tube. The student observed that ton coming in contact with HCl gas, the colour of blue litmus paper:
Aturned yellowBturned violetCremained blueDturned redAssertion (A): Hydrogen chloride gas does not change the colour of dry blue litmus paper.
Reason (R): Hydrogen chloride gas dissolves in the water present in wet litmus paper to form H+ ions.ABoth A and R are true, and R is the correct explanation of A.BBoth A and Rare true, but is not the correct explanation of A.CA is true but R is false.DA is false but R is true.
What is observed when a solution of sodium sulphate is added to a solution of barium chloride taken in a test tube? Write equation for the chemical reaction involved and name the type of reaction in this case.
View Solution1 g of solid sodium chloride is taken in a clean and dry test tube and 2 mL of conc. Suphuric acid is added to it . If the gas evolved is tested first with dry and then with wet blue litmus paper, in which case will the litmus paper change colour? Give reason for your answer. what inference can be drawn about the nature of the evolved gas ? Support your answer with a chemical equation for the reaction.
View SolutionUsing a pair of tongs, hold a piece of magnesium ribbon over a flame and heat it until the ribbon catches fire.Carefully, collect the white ash obtained from the burnt ribbon in a china dish. Dissolve a little bit of this ash in a test tube containing water. Read the following questions and try to answer them
(a). Test this solution with read and blue litmus papers. What do you observe?
(b). What can you conclude about the reaction of metals with oxygen from this experiment?
View Solution2 ml of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolved which is bubbled through a soap solution before testing.
Write the equation of the chemical reaction.
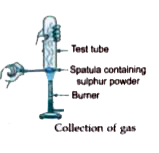
View SolutionPratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it as shown in Fig. below : (a) What will be the action of gas on (i) dry litmus paper ? (ii) moist litmus paper ?
(b) Write a balanced chemical equation for the reaction taking place
View Solution
