An ideal gas system undergoes an isothermal process, then the work done during the process is
A
nRTln(V2V1)
B
nRTln(V1V2)
C
2nRTln(V2V1)
D
2nRTln(V1V2)
Text Solution
The correct Answer is:A
|
Similar Questions
Knowledge Check
An ideal gas undergoes isothermal expansion at constant pressure . During the process.
AEnthalpy remains constant but entropy increasesBEnthalpy decreases but entropy increasesCEnthalpy increases but entropy decreasesDBoth enthalpy and entropy remain constantIn an isothermal process for ideal gas.
Aq=0 and ΔE=0Bq≠0 and ΔE=0Cq=0 and ΔE≠0Dq≠0 and ΔE≠0For an ideal gas, in an isothermal process
AHeat content remains constantBHeat content and temperature remain constantCTemperature remains constantDNone of the above
A gas is taken through an isothermal process as shown.Find the work done by the gas
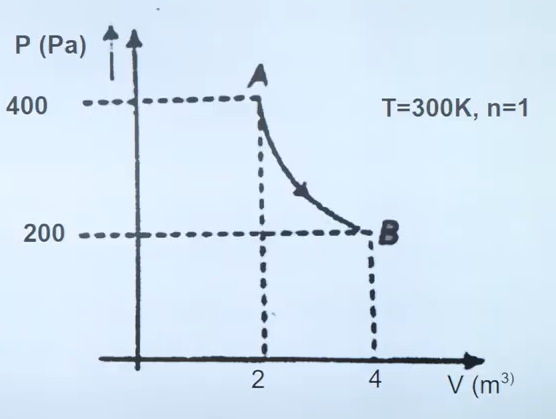
View SolutionAn ideal gas undergoes isothermal expansion at constant pressure . During the process.
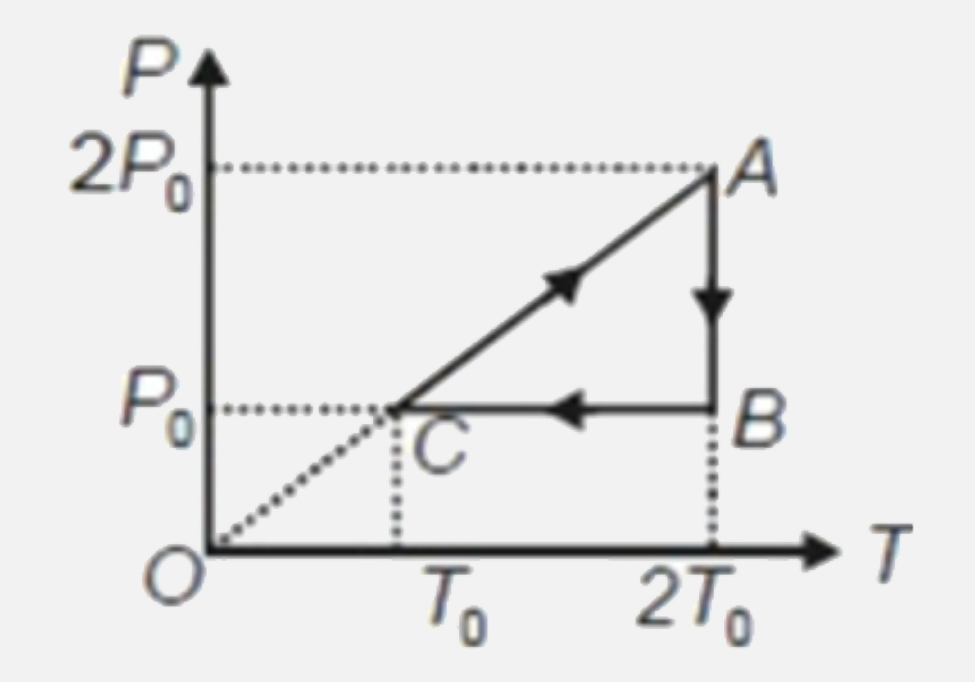
View SolutionThree moles of an ideal gas undergo a cyclic process shown in figure. The work done by the gas during the process is [Take ln (2)=0.693]
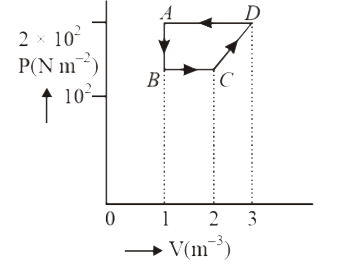
View SolutionThe P-V diagram of a gas system undergoing cyclic process is shown here. The work done during isobaric compression is
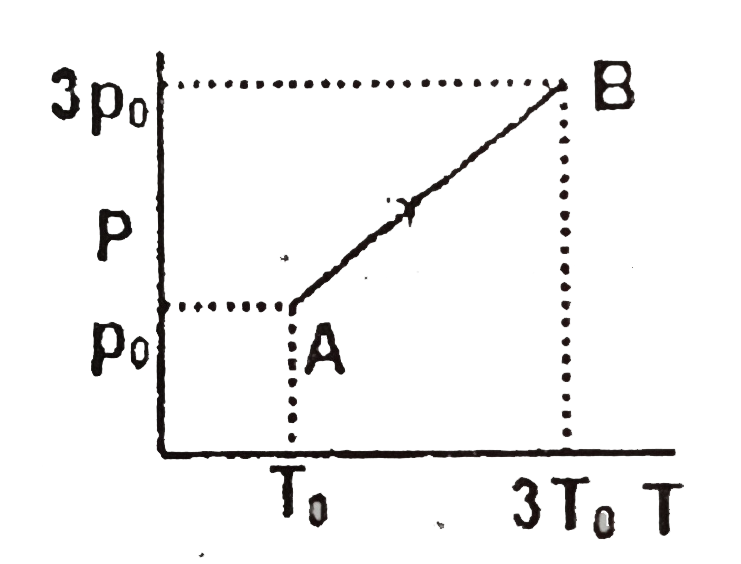
View SolutionTwo moles of a monoatomic ideal gas undergoes a process AB as shown in the figure. Then, find the work done during the process in Joule.
View Solution

