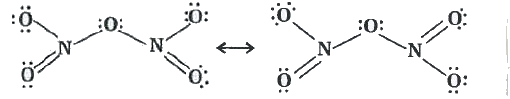
Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.Solution in Gujrati
4HNO3+P4O10→2N2O5+4HPO3
Similar Questions
Nitric acid forms an oxide of nitrogen on reaction with P_4O_(10) Write the reaction involved. Also write the resonating structures of the oxide of nitrogen formed.
View SolutionWhat is the ratio of product gases, nitric oxide and nitrogen dioxide formed when phosphorus is treated to nitric acid?
View SolutionNitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved . Also write the resonating structures of the oxide of nitrogen formed.
View SolutionIn nitric oxide (NO), the oxidation state of nitrogen is
View Solutionनाइट्रिक अम्ल P4O10 से अभिक्रिया करके नाइट्रोजन का एक ऑक्साइड बनाता है। इससे संबंधित अभिक्रिया लिखिए। बनने वाले नाइट्रोजन के ऑक्साइड की अनुनादी संरचनाएँ भी दीजिए।
View SolutionIn nitric oxide (NO) the oxidation state of nitrogen is :
View SolutionNitrogen forms number of oxides in the different oxidation states. Write the names and structural formulae of any four oxides of nitrogen.
View Solution

