What is the product of reaction between excess xenon and fluorine at 673 K temperature ?Solution in Gujrati
XeF2
XeF4
XeF6
Not given
The correct Answer is:A
Step by step video, text & image solution for What is the product of reaction between excess xenon and fluorine at 673 K temperature ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Xenon Fluorides : Xenon reacts directly with fluorine on heating at 675 K in a sealed nickel tube. The product obtained depends upon the amount of fluorine present. XeF_2 reacts with SbF_5 to form
View SolutionXenon Fluorides : Xenon reacts directly with fluorine on heating at 675 K in a sealed nickel tube. The product obtained depends upon the amount of fluorine present.
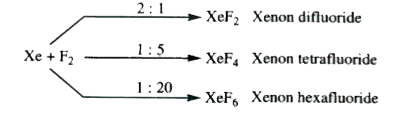
XeF6 on complete hydrolysis gives
View SolutionWhat are the products formed in the reaction of xenon hexafluoride with silicon dioxide?
View SolutionWhat are the products formed in the reaction of xenon hexafluoride with silicon dioxide ?
View SolutionWhat are the products formed in the reaction of xenon hexafluoride with silicon dioxide?
View SolutionXenon react with fluorine to form 3 types of fluorides. When Xe treated with F_2 (2 : 1 ratio) at 673 K and 1 bar pressure give:
View SolutionWhen propane is heated with excess of Cl_2 at 573-673 K under 75-100 atm. Pressure, the products obtained are:
View Solution

