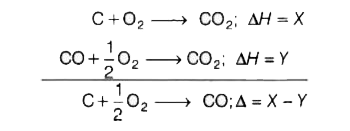
यदि C(s)+O2(g)→CO2(g)ΔH=X तथा CO(g)+12O2(g)→CO2(g),ΔH=Y तब CO की सम्भवन ऊष्मा है
X+Y
X-Y
Y-X
XY
The correct Answer is:B
Similar Questions
C(s)+O_(2)(g)toCO_(2)(g),DeltaH=-94 kcal 2CO(g)+O_(2)to2CO_(2),DeltaH=-135.2 kcal The heat of formation of CO(g) is
View SolutionOn the basis of following thermochemical equations H_(2)O(g)+C(s)toCO(g)+H_(2)(g),DeltaH=131kJ CO(g)+(1)/(2)O_(2)(g)toCO_(2)(g),DeltaH-282kJ H_(2)(g)+(1)/(2)O_(2)(g)toH_(2)O(g),DeltaH=-242kJ C(s)+O_(2)(g)toCO_(2)(g),DeltaH= X kJ:- The value of X will be
View SolutionWhy are the standard reaction enthalpies of the following two reactions different? (1) C(graphite, s) +O_(2)(g)toCO_(2)(g),DeltaH^(0)=-393.5kJ (2) C(diamond, s) +O_(2)(g)toCO_(2)(g),DeltaH^(0)=-395.4kJ
View SolutionGiven: C(s)+O_(2)(g)toCO_(2)(g),DeltaH=-393.5kJ 2H_(2)(g)+O_(2)(g)to2H_(2)O(g),DeltaH=-571.6kJ Calculate DeltaH of the reaction: C(s)+2H_(2)O(g)toCO_(2)(g)+2H_(2)(g) .
View SolutionCalculate DeltaH of the following reaction at 25^(@)C . 4Fe(s)+3O_(2)(g)to2Fe_(2)O_(3)(s) Given: Fe_(2)O_(3)(s)+3C("graphite")to2Fe(s)+3CO(g),DeltaH=117.30kcal C(graphite) +O_(2)(g)toCO_(2)(g),DeltaH=-94.05kcal CO(g)+(1)/(2)O_(2)(g)toCO_(2)(g),DeltaH=67.63kcal at 25^(@)C .
View SolutionConsider the following reactions: C(s)+O_(2)(g)toCO_(2)(g)+x " kJ" CO(g)+(1)/(2)O_(2)(g)toCO_(2)(g)+y" kJ" The heat formation of CO(g) is :
View SolutionC(s)+O_(2)(g)toCO_(2)(g),DeltaH=-94 kcal 2CO(g)+O_(2)to2CO_(2),DeltaH=-135.2 kcal The heat of formation of CO(g) is
View Solution