An organic compound A(C4H7C13) yields(B) when treated with aq. KOH. (B) upon treatment with C2H5OH in presence of acid gave (C) which upon reducing with LiAIH4 gave (D) and (E). (B) upon treatment with NH3 followed by heating with P4O10 and subsequent hydrolysis gives back(B). Sodium salt of (B) on Kolbe's electrolysis gave 2, 3-dimethylbutane at anode.
Compound (B) can also be obtained by Solution in Telugu
catalysed (HgSO4) hydration of 1-butone
reduction of CH3−OH∣CH−O∣∣COC2H5 with LiAIH4
oxidative cleavage of 4-methyl-2-pentene
All the above
The correct Answer is:C
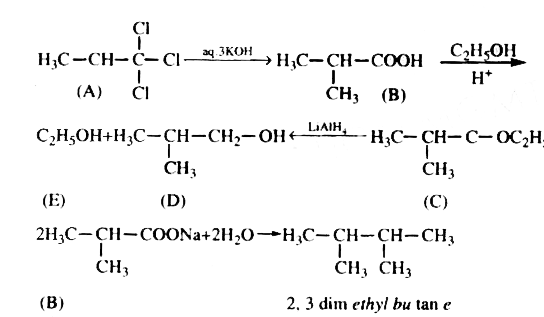
oxidative cleavager of 4-methyl 2-pentene gives acetic acid and isobutylic acid (B) H3C−HC=C∣CH3H−CH−CH3KMnO4−−−−→H+H3C−COOH+H∣CH33C−CH−COOHB
Topper's Solved these Questions
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:LEVEL-II LEXTURE SHEET (Exericse-III) (Match the following questions)
Explore 5 Videos
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:LEVEL-II LEXTURE SHEET (Exericse-IV) (Integer answer type Questions)
Explore 9 Videos
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:LEVEL-II LEXTURE SHEET (Exericse-I) (Single or more than one option questions)
Explore 21 Videos
- CARBOHYDRATESBook:AAKASH SERIESChapter:CARBOHYDRATESExercise:Practice Sheet-5
Explore 30 Videos
- CARBOXYLIC ACIDS AND DERIVATIVESBook:AAKASH SERIESChapter:CARBOXYLIC ACIDS AND DERIVATIVESExercise:CONVERSIONS
Explore 19 Videos
Similar Questions
Match the following question
View SolutionNo of positional isomers possible for benzene dicarboxylic acid
View SolutionThe increasing order of acidity :
View SolutionD-tagatose is a 2-ketohexose which forms same osazone as D-galactose. What is the structure of D-tagatose
View SolutionMethanol to acetic acid
View SolutionAn orgnic compound (A) on reduction gave a compound (B). Upon treatment with HNO2 ,(B) gave ethyl alcohol and on warming with CHCI3, and alcoholic KOH, (b) gave offensive smell. The compound (A) is:
View SolutionAn organic compound 'A' having molecular formula C2H3N on reduction gave another compound 'B' Upon treatment with nitrous acid, 'B' gave ethyl alcohol on warming with chloroform and alcoholic KOH, it formed an offensive smelling compound 'C'. The compound 'C' is :
View SolutionCompound A reacts with PCl5 to give B which on treatment with KCN followed by hydrolysis gave propionic acid. What is A and B respectively?
View SolutionAn organic compound A having molecular formula C2H3N on reduction gave a compound B. Upon treatment with HONO,B gave ethyl alcohol and on warming with CHCI3 and alcoholic KOH , it gave offensive smell. The compound A is
View SolutionCompound A reacts with PCI, to give B which on treatment with KCN followed by hydrolysis gave propanoic acid. What is A and B respectively?
View SolutionAn organic compound ‘A’ on treatment with KCN gave B which on hydrolysis with dil. HCl gave acetic acid. Identify A.
View SolutionAn organic compound A having molecular formula C_(2)H_(3)N on reduction gave a compound B. Upon treatment with HONO, B gave ethyl alcohol and on warming with CHCI_(3) and alcoholic KOH, it gave offensive smell. The compound A is:
View SolutionCompound A reacts with PCI_5 to give B which on treatment with KCN followed by hydrolysis gave propionic acid. What are A & B respectively?
View SolutionAn organic compound A(C4H7C13) yields(B) when treated with aq. KOH. (B) upon treatment with C2H5OH in presence of acid gave (C) which upon reducing with LiAIH4 gave (D) and (E). (B) upon treatment with NH3 followed by heating with P4O10 and subsequent hydrolysis gives back(B). Sodium salt of (B) on Kolbe's electrolysis gave 2, 3-dimethylbutane at anode.
Structural formula of compound (C) is
View SolutionAn organic compound A(C4H7C13) yields(B) when treated with aq. KOH. (B) upon treatment with C2H5OH in presence of acid gave (C) which upon reducing with LiAIH4 gave (D) and (E). (B) upon treatment with NH3 followed by heating with P4O10 and subsequent hydrolysis gives back(B). Sodium salt of (B) on Kolbe's electrolysis gave 2, 3-dimethylbutane at anode.
Compound (D) and (E) respectively are
View Solution
