Hydroxy ethanoic acid is a white solid, readly soluble in water. It is prepared by the hydrolysis of chloroethanoic acid with sodium hydroxide followed by acidification. It exhibits the properties of both a monocarboxylic acid and a primary alchol. In addition, it undergoes reactions which neither a carboxylic acid nor a primary alcohol can undergo.
Which of the following reagent can convert 2-hydroxy propanoic acid to 2-oxo propanoic acid? Solution in Telugu
Ag2O
Na2Cr2O7/H2SO4
Conc. H2SO4
Br2/CCl4
The correct Answer is:A
Step by step video, text & image solution for Hydroxy ethanoic acid is a white solid, readly soluble in water. It is prepared by the hydrolysis of chloroethanoic acid with sodium hydroxide followed by acidification. It exhibits the properties of both a monocarboxylic acid and a primary alchol. In addition, it undergoes reactions which neither a carboxylic acid nor a primary alcohol can undergo. Which of the following reagent can convert 2-hydroxy propanoic acid to 2-oxo propanoic acid? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:PRACTICE SHEET-3 (Matching Type Questions)
Explore 2 Videos
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:PRACTICE SHEET-3 (Integer Type questions)
Explore 5 Videos
- CARBOXYLIC ACIDS Book:AAKASH SERIESChapter:CARBOXYLIC ACIDS Exercise:PRACTICE SHEET-3 (More than one correct answer questions)
Explore 4 Videos
- CARBOHYDRATESBook:AAKASH SERIESChapter:CARBOHYDRATESExercise:Practice Sheet-5
Explore 30 Videos
- CARBOXYLIC ACIDS AND DERIVATIVESBook:AAKASH SERIESChapter:CARBOXYLIC ACIDS AND DERIVATIVESExercise:CONVERSIONS
Explore 19 Videos
Similar Questions
Matching Type questions
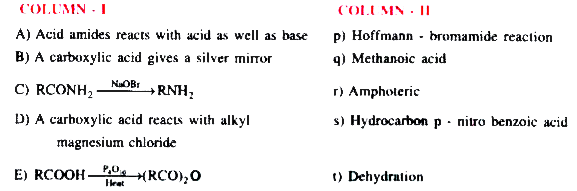
View SolutionHow many of the following are stronger than HCOOH (formic acid) ?
Ph−COOh,Ph,CH−COOH,CH3−COOH,I−CH2−COOH,
Br−CH2−COOH,Cl−CH2−COOH,F−CH2−COOH,NC−CH2−COOH
O2N−CH2−COOH,H2C=CH−COOH
View SolutionWhich of the following acids are dicarboxylic acids?
View SolutionD-tagatose is a 2-ketohexose which forms same osazone as D-galactose. What is the structure of D-tagatose
View SolutionMethanol to acetic acid
View Solutionपरिवर्तित करें--- एथेनोइक अम्ल को प्रोपेनोइक अम्ल में
View Solution2 - हाइड्रोक्सी-प्रोपेनोइक अम्ल संरचना सूत्र बनाओ ?
View SolutionHydroxy ethanoic acid is a white solid, readly soluble in water. It is prepared by the hydrolysis of chloroethanoic acid with sodium hydroxide followed by acidification. It exhibits the properties of both a monocarboxylic acid and a primary alchol. In addition, it undergoes reactions which neither a carboxylic acid nor a primary alcohol can undergo. Which of the following products is formed when hydroxyethanoic acid is being heated?
View SolutionHydroxy ethanoic acid is a white solid, readly soluble in water. It is prepared by the hydrolysis of chloroethanoic acid with sodium hydroxide followed by acidification. It exhibits the properties of both a monocarboxylic acid and a primary alchol. In addition, it undergoes reactions which neither a carboxylic acid nor a primary alcohol can undergo.
Which of the following ractions is NOT given by hydroxy ethanoic acid ?
View SolutionHydroxy ethanoic acid is a white solid, readly soluble in water. It is prepared by the hydrolysis of chloroethanoic acid with sodium hydroxide followed by acidification. It exhibits the properties of both a monocarboxylic acid and a primary alchol. In addition, it undergoes reactions which neither a carboxylic acid nor a primary alcohol can undergo. Hydroxyl ethanoic acid can be prepared by
View SolutionEthanoic acid to propanoic acid
View SolutionEthanoic acid to propanoic acid
View SolutionHow will you convert Ethanoic acid to Propanoic Acid ?
View SolutionHow will you convert : Ethanoic acid into propanoic acid
View SolutionHow will you convert :
Propanoic acid into ethanoic acid ?
View Solution
