For an exothermic chemical process occurring in two steps as
(i) A+B→X (slow) (ii) X→AB (fast)
The progress of the reaction can be best described by
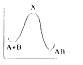
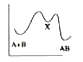
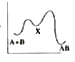
All are correct
The correct Answer is:B
Topper's Solved these Questions
- CHEMICAL KINETICSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETICSEXERCISE - LEVEL-1 (EXERCISE -I ORDER MOLECULARIT AND HALF LIFE:)
45 Videos
- CHEMICAL KINETICSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETICSEXERCISE - LEVEL-1 (EXERCISE -I ASSERTION REASON TYPE:)
9 Videos
- CHEMICAL KINETICSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETICSEXERCISE - Objective Exercise - 4 (Assertion (A) & Reason (R) Type Questions)
44 Videos
- CHEMICAL KINETCSBOOK - AAKASH SERIESCHAPTER - CHEMICAL KINETCSEXERCISE - EXERCISE - 3.2
45 Videos
- CHEMICAL THERMODYNAMICSBOOK - AAKASH SERIESCHAPTER - CHEMICAL THERMODYNAMICSEXERCISE - Additional Practice Exercise
54 Videos
Similar Questions
Which of the foloiwng is correct?
View SolutionA: Spontaeous reaction may be slow or fast.
R: Spontanteous nature deals with feasibility of the reaction but not rate.
View Solution(A) Spontaneous reaction may be slow or fast.
(R ) Spontaneous nature deals with feasibility of the reaction but not rate.
View SolutionDiscuss on the kinetic spontaneity of a chemical reaction. Is a slow reaction spontaneous? Why?
View SolutionWhich among the following is not an exact differential?
View SolutionFor an exothermic chemical process ocuuring in two process occuring in two steps as follows (i)A+BtoX("slow")" "(ii)XtoAB("fast") The progress of reaction can be best described by :
View SolutionThe following reaction is accompanied by liberation of heat and occurs in two steps as follows
i) R−X→R++X− (slow)
ii) R++Nu−→R−Nu (fast)
The progress of the reaction is best represented by
View SolutionFor an exothermic chemical process occuring in 2 steps as (i) A+B rarr X (slow) , (ii) X rarr AB (fast) The progress of the reaction can be best described by (x - intermediate).
View SolutionFor an exothermic chemical process ocuuring in two process occuring in two steps as follows
(i)A+B→X(slow) (ii)X→AB(fast)
The progress of reaction can be best described by :
View SolutionA chemical reaction proceeds into the following steps
Step I, 2A⇔X fast
Step II, X+B→Y slow
Step III, Y+B⇔ product fast
The rate law for the overall reaction is
View SolutionFor an exothermic reaction, following two steps are involved.
Step 1. A+B→I (slow)
Step 2. I→AB (fast)
Which of the following graphs correctly represent this reaction ?
View SolutionFor an exothermic chemical process ocuuring in two process occuring in two steps as follows
(i)A+B→X(slow) (ii)X→AB(fast)
The progress of reaction can be best described by :
View SolutionAn exothermic chemical process occurs in the following two terms (i) A + B to X (Slow) , (ii)X to AB (fast) The process of reaction can be best explained by
View SolutionFor an exothermic chemical process occuring in two steps as : A+BrarrX(slow) , A+BrarrX(slow) The progress of the reaction can be described by :
View SolutionFor an exothermic chemical process occuring in two steps as : A+BrarrX(slow) , A+BrarrX(slow) The progress of the reaction can be described by :
View Solution

