Select the correct statements(s)Solution in Telugu
The rare law of the elementary reaction 2A→B+C, must bne Rate =K[A]2
The rate law for the complex reaction A+B→C, might not be Rate =K[A][B]
If the partial orders differ from the coefficients in the balanced reaction, the reaction must be complex.
If the partial orders equal to corresponding coefficients in the balanced reaction, the reaction must be elementary.
The correct Answer is:A, B, C
If partial orders equal to thecoefficients of balanced eqn. then the reaction may be simple on complex.
Topper's Solved these Questions
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:LEVEL-II(LECTURE SHEET EXERCISE-II LINKED COMPREHENSION TYPE QUESTIONS)
Explore 9 Videos
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:LEVEL-II(LECTURE SHEET EXERCISE-III MATCH THE FOLLOWING QUESTIONS)
Explore 3 Videos
- CHEMICAL KINETICSBook:AAKASH SERIESChapter:CHEMICAL KINETICSExercise:LEVEL-1 (EXERCISE -II ORDER, MOLECULARITY, HALF LIFE:)
Explore 34 Videos
- CHEMICAL KINETCSBook:AAKASH SERIESChapter:CHEMICAL KINETCSExercise:EXERCISE - 3.2
Explore 45 Videos
- CHEMICAL THERMODYNAMICSBook:AAKASH SERIESChapter:CHEMICAL THERMODYNAMICSExercise:Additional Practice Exercise
Explore 54 Videos
Similar Questions
A certain endothermic reaction: A→ Product, ΔH=+ve proceeds ina sequence of three elementary steps with the rate constant K1,K2 and K3 and each one having energy of activation E1,E2 and E3 respectively at 25∘C. The observed rate constannt for the reaction is equal to K3√K1K2.
A1,A2 and A3 are Arrhenius parameters respectively.
The observed energy of activation for the reaction is
View SolutionMatch the following columns
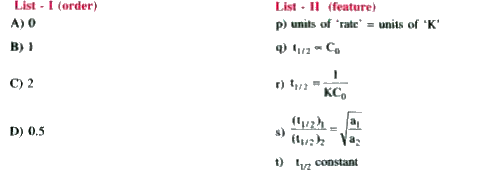
View SolutionSucrose decompose in acid solution into glucose and fructose according to the first order rate law, with t12=3.00 hours. What fraction of sample of sucrose remains after 8 hours ?
View SolutionDiscuss on the kinetic spontaneity of a chemical reaction. Is a slow reaction spontaneous? Why?
View SolutionWhich among the following is not an exact differential?
View SolutionSelect the correct statement (s).
View SolutionSelect the correct statement (s) .
View SolutionSelect the correct statement (s)
View SolutionSelect the correct statement (s) .
View SolutionSelect the correct statement (s)
View SolutionSelect the correct statements (s).
View SolutionSelect the correct statements (s).
View SolutionSelect correct statement(s).
View SolutionSelect correct statement(s)
View SolutionSelect correct statement(s).
View SolutionSelect correct statements(s).
View Solution
