The following data re obtained from the decomposition of a gaseous compound

The order of the reaction is Solution in Telugu
A
0.5
B
1
C
1.5
D
2
Video Solution
Text Solution
The correct Answer is:C
(t12)1(t12)2=(P2P1)n−1,(80113)=(816)n−1=(80113)=(12)(n−1)(11380)=(21)(n−1),1.4125=2(n−1)
log1.4125=(n−1)log2,n=(0.498+1)=1.498=1.5
|
Similar Questions
Match the Column

View SolutionThermal decomposition of gaseous ammonia is a zero order reaction. What is the catalyst in this case?
View SolutionIn the first order decomposition of oxalic acid following data were obtained.

The half-life period of the reaction is_____________ min.
View SolutionThe following data are for the decomposition of ammonium nitrate in aqueous solution :
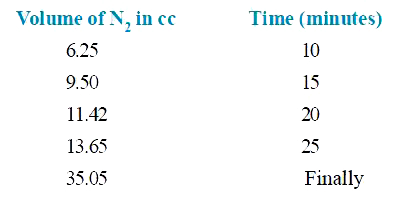
The order of the reaction is:
View SolutionFollowing data were obtained for the catalytic decomposition of ammonia : Find the order of reaction.
View SolutionFollowing data were obtained for the catalytic decomposition of ammonia :
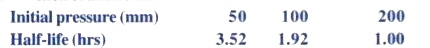
Find the order of reaction.
View SolutionFollowing data were obtained for the catalytic decomposition of ammonia :
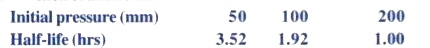
Find the order of reaction.
View Solution

