(a) Explain SN1 mechanism for the conversion of tertiary butyl bromide to tertiary butyl alcohol.
(b) Complete the following reactions :
(i) CH3−CH=CH2+HI→
(ii) 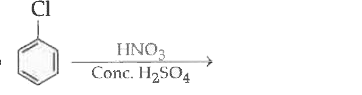
(iii) CH3CH2BrAgCN−−−−−−→Aq EthanolSolution in Kannada
In this step, polarised C Br bond undergoes cleavage to produce a carbocation and a bromide ion.
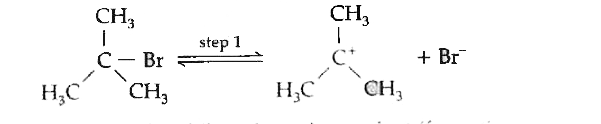
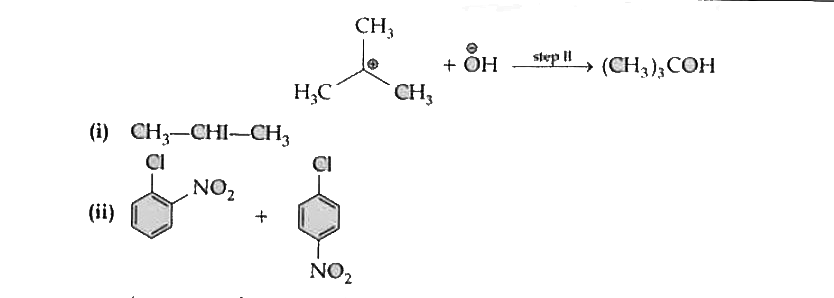
In this step, carbocation is attacked by nucleophile to form the product (formation of C-OH bond).
(b) (i) CH3−CHI−CH3
(ii) 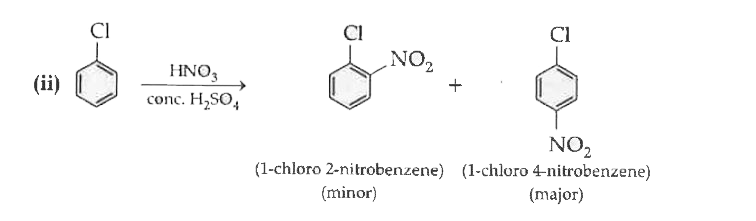
(iii) CH3CH2NC
(a) Conversion of tertiary butyl bromide to tertiary butyl alcohol using SN1 mechanism occurs as follows :
Step - 1 : Formation of carbocation :
CH3−CH3∣C∣CH3−Br(tertiary butyl bromide)slow step−−−−→CH3−CH3∣C+∣CH3(carbocation)+Br−
Step - 2: Fast step and formation of racemic mixture of product :
CH3−CH3∣C+∣CH3+OH−(Nucleohile)fast−−→CH3−CH3∣C∣CH3−OH(tertiary butyl alcohol)
(b) (i) This is an example of unimolecular nuclephile substitution reaction i.e. rate ∝ [substrate]
(b) (i) CH3−CH=CH2+HI→CH3−CH∣I−CH3
(ii) 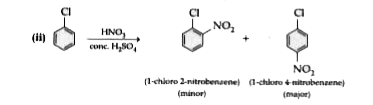
(iii) CH3.CH2.BrAgCN−−−−→aq.thanolCH3.CH2.NC(etyl isocynaide)+AgBr
Similar Questions
Identify the most suitable reagent for the following conversion ? CH_(3)-CH_(2)-underset(Br)underset(|)(CH)-CH_(3)overset("Reagent")(rarr)CH_(3)-CH=CH-CH_(3)
View SolutionWhich alkyl halide from the following pairs would you expect to react more rapidly by SN2 mechanism ? Explain your answer.
CH3CH2CH2CH2 or CH3CH2−C∣BrH−CH3
(ii) CH3CH2C∣BrHCH3 or H3C−CH3∣C∣CH3−Br
(iii) CH3C∣CH3HCH2CH2Br or CH3CH2C∣CH3HCH2Br
View SolutionClassify the following as primary, secondary and tertiary alcohols:
(i) CH3−CH3| C| CH3−CH2OH (ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
(iv)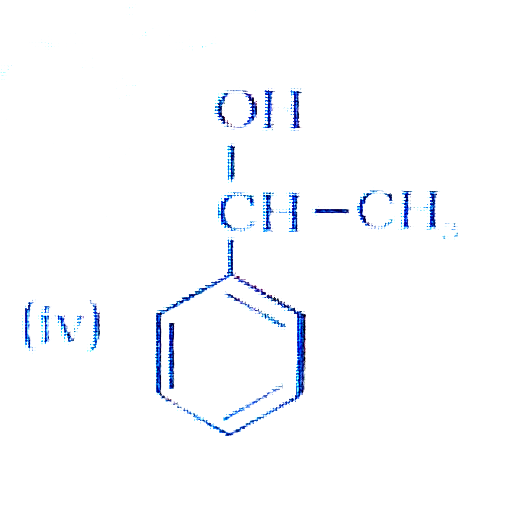
View SolutionClassify the following as primary alcohols, secondary alcohols and tertiary alcohols.
(i) CH3−CH3∣C∣CH3−CH2OH
(ii) H2C=CH−CH2OH
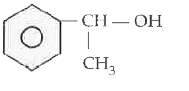
(iv) CH3−CH2−CH2−OH
View SolutionClassify the following as primary, secondary and tertiary alcohols :
(i) CH3−CH3| C | CH3−CH2OH
(ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
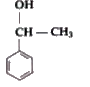

View SolutionIdentify the most suitable reagent for the following conversion ? CH_(3)-CH_(2)-underset(Br)underset(|)(CH)-CH_(3)overset("Reagent")(rarr)CH_(3)-CH=CH-CH_(3)
View SolutionThe product "P" of the following reaction is : CH_(3)-CH_(2)-overset(CH_(3))overset(|)CH-underset(ONa)underset(|)(CH)-CH_(3) + CH_(3)-CH_(2)-Br rarr P
View Solution
