Compose an activity to arrange Ca, Mg and Fe metals in the decreasing order of reactivity with water. Write suitable balanced chemical equations and draw diagrams.Solution in Kannada
Take some metals like Ca, Mg and Fe etc. Place separately small pieces of these metals in beakers with small amount of water in them. If the metals react with the cold water, then the reaction is vigorous. If metals does not react with cold water, then treat it with hot water. Record the observations and arrange the metals in decreasing order of reactivity.
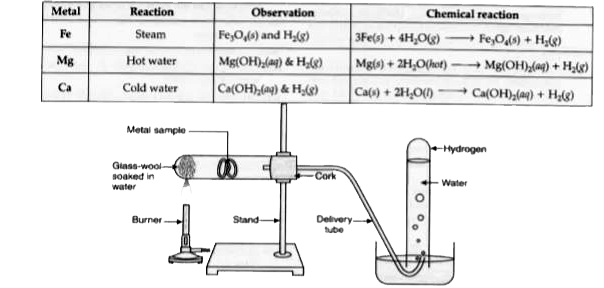
After this activity it can be concluded that Ca is more reactivity than Mg, which is more creative than Fe.
Similar Questions
Arrange the metals such as Al, Cu, Fe, Pb, Mg, Zn and Na in the decreasing order of their reactivity towards oxygen.
View SolutionArrange the given metals (in activity) in the decreasing order of their reactivity with water.
View SolutionArrange the metals Mg, Al, Zn and Fe in the decreasing order of their reactivity towards dilute hydrochloric acid.
View SolutionArrange the metals: Fe, Na, Ag and Zn in Increasing order of their chemical reactivity
View SolutionArrange the following metals in the order of their decreasing reactivity, Fe, Cu, Mg, Ca, Zn, Ag
View SolutionZn, AL,Mg एवं Fe दिए गए धातुओं को घटते अभिक्रियात्मकता क्रम में लगाएँ।
View SolutionAnswer the following questions:
Arrange the following metals in the decreasing order of chemical reactivity: Cu, Mg, Fe,Ca, Zn, Na.
View SolutionArrange Na, Mg , Fe, Pb , Hg and Cu in order of decreasing reactivity.
View Solution