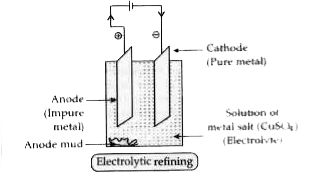
Describe electrolytic refining of copper with chemical equations. Draw a well as labelled diagram for it.Solution in Kannada
Electrolytic Refining : This method is widely used as purification of metals like zinc (Zn), copper (Cu), aluminium (Al), chromium (Cr), tin (Sn), lead (Pb), nickel (Ni) and gold (Au). In this process, impure metal is used as anode, a strip of pure metal is used as cathode and soluble salt of metal is used as electrolyte. On passing electric current through the electrolyte, cations move towards cathode, gain electrons and pure metal deposited on cathdoe. In electrolytic refining of copper the impurities left behind at anode called anode and contains valuable metals such as gold and silver which can be recovered in the native state
Similar Questions
Draw the diagram of the apparatus used in electrolytic refining of copper and label the electrode where pure copper is deposited.
View SolutionWhat is meant by refining of metals? Describe the electrolytic refining of copper with a neat labelled diagram.
View SolutionDraw the diagram of an electrolytic cell used in the purification of copper and label in the purification of copper and label the electrode having impure copper.
View SolutionGiven below are the steps for extraction of copper from its ore. Write the reaction involved.
(i) Roasting of copper (I) sulphide.
(ii) Reduction of copper (I) oxide with copper (I) sulphide.
(iii) Electrolytic refining
(b) Draw a neat and well labelled diagram for electrolytic refining of copper.
View Solution(a) Explain the steps for extraction of copper from its ore. Write the reaction involved.
(b) Draw a neat, labelled diagram for electrolytic refining of copper and explain the process.
View SolutionExplain the electrolytic refining of copper with the help of a diagram .
View SolutionHow is impure copper purified by electrolytic refining? Draw a neat labelled diagram to illustrate it.
View Solution
