Illustrate the formation of bond in :
(i) Sodium chloride (ii) Magnesium chloride.
Identify the ions present in these compounds.Solution in Kannada
(i) Na−−→Na++e−2,8,12,8
Cl+e−−→Cl−2,8,72,8,8
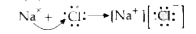
(ii) (Mg,→,Mg2+,+2e−,),(2,8,2,,2,8,),(Cl+e,→,Cl,),(2,8,7,,(2,8,8),)
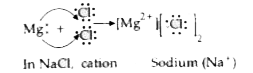
In NaCl, cation−Sodium (Na+) anion−Chloride (Cl−)In MgCl2 cation−Magnesium (Mg2+) anion−Chloride (Cl−)
Similar Questions
(a) Show the formation of magnesium chloride and sodium chloride by transfer of electrons. (b) Identify the ions present in these compounds. (c) Why do ionic compounds not conduct electricity in the solid state?
View Solution(i) By the transfer of electrons, illustrate the formation of bond in magnesium chloride and identify the ions present in this compound.
(ii) Ionic compounds are are solids. Give reasons.
(iii) With the help of a labelled diagram show the experimental set up of action of steam on a metal.
View SolutionStatement I Sodium chloride is a permanent compound produced as a result of the action of chlorine gas on sodium metal. Statement II This is because sodium and chloride complete their octaves in the process of sodium chloride formation.
View SolutionFor a crystal of sodium chloride, state :
The number of sodium ions and chloride ions present in unit cell of sodium chloride.
View SolutionFor a crystal of sodium chloride, state: (i) The type of lattice in which it crystallizes. (ii) The coordination number of each sodium ion and chloride ion in the crystal lattice. (iii) The number of sodium ions and chloride ions present in a unit cell of sodium chloride. (iv) The structural arrangement of the sodium chloride crystal.
View Solutionസോഡിയം ക്ലോറൈഡ് രൂപീകരിക്കുന്നത് അയോണീക ബന്ധനം സ്ഥിരീകരിക്കുക ?
View SolutionFor crystal sodium chloride, state
(i) the type of lattice in which it crystallises.
(ii) co-ordination number of each sodium ion and chloride ion in the crystal lattice.
(iii) number of sodium and chloride ions present in a unit cell of sodium chloride.
(iv) the structural arrangement of sodium chloride crystals.
View SolutionIdentify the metals present in the following compounds (i) Potassium chloride (ii) Calcium nitrate (iii) Aluminium chloride
View Solution