(i) Write the electron-dot structures for sodium (11) oxygen (8), chlorine (17) and magnesium (12) show the formation of Na2O and MgO by the transfer of electrons.
(ii) Name the ions present in these compounds.
(i) Sodium (Na) - 2,8,1 ∗Na
Chlorine (Cl) - 2,8,7 .⋅..C..l:
Oxygen (O) - 2,6 :..O:
Magnesium (Mg) - 2,8,2 Mg:
Mg−−→Mg2++2e−2,8,22,8(Magnesium cation)O+2e−−−→O2−2,62,8
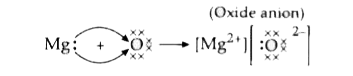
Na−−→Na++e−2,8,12,8O+2e−−−→O2−2,62,8
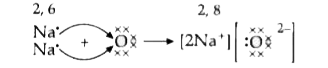
(ii) In MgO, cation - Mg2+, Anion - O2
In Na2O, cation - Na+, Anion - O2
Similar Questions
Show the formation of Na2OandMgO by the transfer of electrons.
View Solution(a) Show the formation of Na2O and MgO by the transfer of electrons.
(b) What are the ions present in these compounds?
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium. (ii) Show the formation ofNa2O and MgO by the transfer of electrons. (iii) What are the ions present in these compounds ?
View Solution(i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2OandMgO by the transfer of electrons.
View SolutionWrite the electron dot structures of sodium, oxygen and magnesium.
View Solution(i) Write electrondot structures for magnesium and oxygen. ltBrgt (ii) show the formation of MgO by the transfer of electrons. ltBrgt (iii) . What are the ions present in this compound?
View Solution(a) Write the electron dot structure for calcium and oxygen. The atomic numbers of calcium and oxygen are 20 and 8 respectively.
(b) Show the formation of calcium oxide by the transfer of electrons.
(c) Ionic compounds are high melting solids. Give reason.
View Solution
