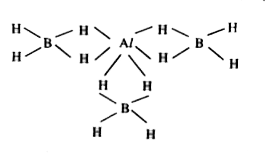
Al[BH4]3 contains how many 3 centered −2e− bonds. Solution in Telugu
Video Solution
Text Solution
The correct Answer is:6
|
Similar Questions
Identify the correct statements regarding the structure of Al(BH4)3.
1. Al is sp3d2andB is sp3 hybridized
2. It has 6 3c−2e− bonds
3. It has 6 Al - H - B bonds
4. It has 6 2c−2e− bonds.
View SolutionAl+3 has how many protons?
View SolutionDiborane is heated with CO at 100∘C and 20 atmospheres to form
(A) 3[BH3⋅CO]
(B) 2[BH3⋅CO]
(C) 4[BH3⋅CO]
(D) 1[BH3⋅CO]
View SolutionTri alkyl aluminium molecules exists as dimer which contains 3 centered 2e bonds. The coordination number of bridged carbon atoms is
View SolutionIdentify the correct statements regarding the structure of Al(BH4)3
View Solutionडाइबोरेन में अधिकतम कितने 2c - 2e बंध है :
View SolutionHow many 3c-2e bonds are present in diborane ?
View Solution