Hardness of water is expressed as the number of parts by weight of present in 10 parts by weight of water Solution in Telugu
CaCl2
CaCO3
MgCl2
CaSO4
The correct Answer is:B
Step by step video, text & image solution for Hardness of water is expressed as the number of parts by weight of present in 10 parts by weight of water by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - OBJECTIVE EXERCISE - 1 (HEAVY WATER)
10 Videos
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - OBJECTIVE EXERCISE - 1 (HYDROGEN PEROXIDE)
19 Videos
- HYDROGEN AND ITS COMPOUNDSBOOK - AAKASH SERIESCHAPTER - HYDROGEN AND ITS COMPOUNDSEXERCISE - OBJECTIVE EXERCISE - 1 (WATER)
3 Videos
- HYDROCARBONSBOOK - AAKASH SERIESCHAPTER - HYDROCARBONSEXERCISE - ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))
5 Videos
- IONIC EQUILIBRIUM BOOK - AAKASH SERIESCHAPTER - IONIC EQUILIBRIUM EXERCISE - ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))
8 Videos
Similar Questions
In hevy water molecule
View SolutionOxygenated water is
View SolutionHydrogens in solid ice each oxygen is surrounded by oxygen in ....position
View SolutionMatch the intermediates formed in the reaction.
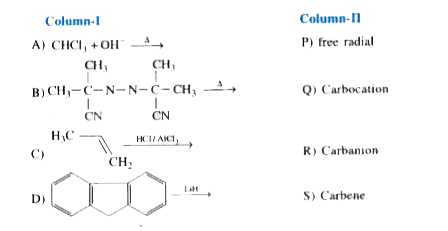
View SolutionA certain weak acid has Ka,=10−5. If the equilibrium constant for is its reaction with a strong base is repesented as y×10y then find the value of y.
View SolutionThe total weight of a piece of wood is 6 kg in the floating state in water its 1/3 part remains inside the water on this floating solid what maximum weight is to be put such that the whole of the piece of wood is to be drowned in the water
View Solutionपूर्णतया जल से भरे एक पात्र का वजन 2.25 किग्रा हैं। पात्र के 0.2 भाग में जल भरा होने पर उसका वजन 0.77 किग्रा है। पात्र के 0.4 भाग में जल पात्र होने पर उसका वजन कितने किग्रा होगा?
View SolutionOrdinary water contain one part of heavy water per 6000 parts by weight. The number of heavy water molecules present in drop of water of volume 0.01 ml is (Density of water is 1gm//ml)
View SolutionOne litre of a sample of hard water contains 1 mg of CaCl2 and 1 mg of MgCl2. Find the total hardness of water in terms of parts of CaCO3 per 106 parts of water by mass.
View Solutionएक बाल्टी जब 57 भाग तक पानी से भरी है तो इसका भार 12 किग्रा तक पानी से भरी है और जब 34 भाग तक पानी से है, तो इसका भार 12.5 किग्रा है। खाली बाल्टी का भार क्या है?
View SolutionCalculate the number of water molecules present in one drop of water which weights 0.18 g.
View SolutionOrdinary water contain one part of heavy water per 6000 parts by weight. The number of heavy water molecules present in drop of water of volume 0.01 ml is (Density of water is 1gm/ml)
View SolutionOridinary water contain one part of heavy water per 6000 parts of water by weight. The number of heavy water molecules present in a drop of water of volume 0.01 mL is (density of water 1 g/mL)
View SolutionHardness of water is expressed as the number of parts by weight of present in 10 parts by weight of water
View SolutionA hard water sample contains calcium chloride only degree of hardness with 200 ppm. Calculate the weight of calcium chloride present in 10 kg of water sample.
View Solution

