The ionisation energy of hydrogen is Solution in Telugu
1312KJmole−1
520KJmol−1
495KJmol−1
1681KJmol−1
The correct Answer is:A
Step by step video, text & image solution for The ionisation energy of hydrogen is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:OBJECTIVE EXERCISE - 2A (WATER, HARD WATER, HEAVY WATER)
Explore 20 Videos
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:OBJECTIVE EXERCISE - 2A (HYDROGEN PEROXIDE)
Explore 31 Videos
- HYDROGEN AND ITS COMPOUNDSBook:AAKASH SERIESChapter:HYDROGEN AND ITS COMPOUNDSExercise:OBJECTIVE EXERCISE - 1 (HYDROGEN PEROXIDE)
Explore 19 Videos
- HYDROCARBONSBook:AAKASH SERIESChapter:HYDROCARBONSExercise:ADDITIONAL PRACTICE SHEET ( LEVEL-II (PRACTICE SHEET (ADVANCED) (MATRIX MATCHING TYPE QUESTIONS)))
Explore 5 Videos
- IONIC EQUILIBRIUM Book:AAKASH SERIESChapter:IONIC EQUILIBRIUM Exercise:ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))
Explore 8 Videos
Similar Questions
How many hydrogen bonded water molecules are associated in CuSO4.5H2O?
View SolutionIn electrolysis of dilute H2SO4, what is liberated at anode in the presence of inert electrode ?
View SolutionOxygenated water is
View SolutionMatch the intermediates formed in the reaction.
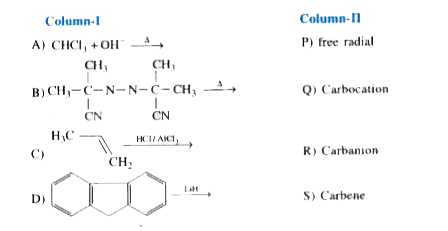
View SolutionA certain weak acid has Ka,=10−5. If the equilibrium constant for is its reaction with a strong base is repesented as y×10y then find the value of y.
View SolutionIf first ionisation energy of hydrogen be E, then the ionisation energy of He+ would be :
View SolutionDefine ionisation energy. What is the value for a hydrogen atom?
View SolutionIonisation energy of hydrogen is
View SolutionProve that the ionisation energy of hydrogen atom is 13.6 eV.
View SolutionThe ionisation energy of hydrogen atom is 13.6eV. What is the ionisation energy of He ?
View SolutionFor ionising an excited hydrogen atom, the energy required (in eV) will be
View SolutionThe ionisation energy of hydrogen is-
View SolutionThe ionisation energy of hydrogen is
View SolutionDefine ionisation energy. What is the value for a hydrogen atom?
View SolutionDefine ionisation energy. What is the value for a hydrogen atom?
View Solution
