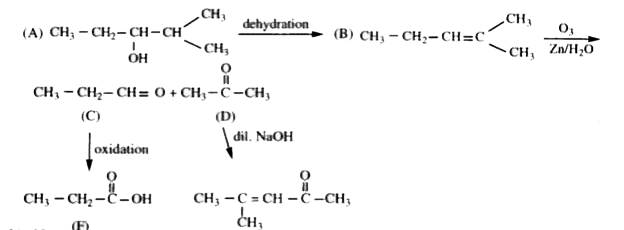
An alcohol (A) on dehydration gives (B) which on ozonolysis gives two products C & D (D) gives positive iodoform test and on heating with dilute alkali gives α.β-unsaturated carbonyl compound (E) which does not gives positive iodoform test. (C) on oxidation gives a mono basic acid (F) Ag salt of (F) contains 59.6% Ag. Oxime of (D) contains 16.09% nitrogen.
the structure of (E ) is given reaction is Solution in Telugu
(CH3)2CHCOCH2CH3
CH3CH2C(CH3)CHCOCH2CH3
CH3CH2CH=CHCOCH2CH3
CH3CH2=C(CH3)CHO
The correct Answer is:A
On zonolysis gives 2 products ∴ By vertification , it is clear that
Topper's Solved these Questions
- ALDEHYDES AND KETONESBook:AAKASH SERIESChapter:ALDEHYDES AND KETONESExercise:PRACTICE SHEET-3
Explore 34 Videos
- ALDEHYDES AND KETONESBook:AAKASH SERIESChapter:ALDEHYDES AND KETONESExercise:Solved Problem
Explore 33 Videos
- ALDEHYDES AND KETONESBook:AAKASH SERIESChapter:ALDEHYDES AND KETONESExercise:PRACTICE SHEET-1
Explore 30 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:AAKASH SERIESChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:ETHERS (OBJECTIVE EXERCISE-4)
Explore 50 Videos
- ALKYL AND ARYL HALIDESBook:AAKASH SERIESChapter:ALKYL AND ARYL HALIDESExercise:OBJECTIVE EXERCISE - 4 (ASSERTION (A) & REASON (R) TYPE QUESTIONS)
Explore 19 Videos
Similar Questions
Which of the following is likely to highest melting point
View SolutionGive the common and IUPAC names of the compound , CH2=CH−CO−CH=CH2
View Solutioncyclohexene CI2/hv−−−−−−−−−−−→Monoha logenetion AalcKOH−−−−→B(1)O3−−−−−−−→(2)Zn/H2O products . The final product in the reaction sequence above
View Solution(A): Alcoholic fermentation involves conversion of sugar into ethanol by the action of yeast.
(R): Fermentation involves the liberation of CO2 gas.
View Solution(A) Towards SN2 reaction, order of reactivity is CH3Br>CH3CH2Br>(CH3)2CHBr>(CH3)3CBr.
(R) Greater the stability of carbocation, greater will be its ease of formation from alkyl halide and faster will be the rate of SN1 reaction.
View SolutionA compound that gives a positive iodoform test is:
View SolutionA compound that gives a positive iodoform test is :
View SolutionWhich of the following alcohols will give the positive iodoform test ?
View SolutionA compound that gives a positive iodoform test is
View SolutionThe compound that gives a positive iodoform test is-
View SolutionThe compound that gives a positive iodoform test is-
View SolutionA compound that gives a positive iodoform test is :
View SolutionA compound that gives a positive iodoform test is
View SolutionWhich of the following compounds on hydrolysis with aqueous alkali give a product which does not give positive iodoform reaction but gives positive silver mirror test. The compound is
View SolutionAn alcohol (A) on dehydration gives (B) which on ozonolysis gives two products C & D (D) gives positive iodoform test and on heating with dilute alkali gives alpha . beta -unsaturated carbonyl compound (E) which does not gives positive iodoform test. (C) on oxidation gives a mono basic acid (F) Ag salt of (F) contains 59.6% Ag. Oxime of (D) contains 16.09% nitrogen. the structure of (A) is
View Solution
