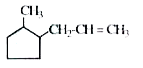
Compounds A(C10H10) decolourises Br, in CCI4 Ozonolysis of A gave B(C10H10,O2). B reacts hydrazine and base followed by heating in ethylene glycol gave a hydrocarbon C(C10H14) C on oxidation gave a dicarboxylic acid which form anhydride easily.
Conversion of B to C is calledSolution in Telugu

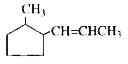

The correct Answer is:D
Step by step video, text & image solution for Compounds A(C_(10) H_(10)) decolourises Br, in C CI_4 Ozonolysis of A gave B(C_(10) H_(10),O_2). B reacts hydrazine and base followed by heating in ethylene glycol gave a hydrocarbon C(C_(10) H_(14)) C on oxidation gave a dicarboxylic acid which form anhydride easily. Conversion of B to C is called by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
Compounds A(C10H10) decolourises Br, in CCI4 Ozonolysis of A gave B(C10H10,O2). B reacts hydrazine and base followed by heating in ethylene glycol gave a hydrocarbon C(C10H14) C on oxidation gave a dicarboxylic acid which form anhydride easily.
the structure of compound 'A' is
View SolutionCompounds A (C_(10) H_(10)) decolourises Br, in C CI_4 Ozonolysis of A gave B (C_(10) H_(10),O_2) . B reacts hydrazine and base followed by heating in ethylene glycol gave a hydrocarbon C (C_(10) H_(14)) C on oxidation gave a dicarboxylic acid which form anhydride easily. Conversion of B to C is called
View SolutionCompounds A(C9H16) decolourises Br, in CCI4 A reacts alkaline KMnO4 to give a diol. A on ozonolysis gave B(C7H12O) and acetaldehyde. B reacts in presence of sodium hydroxide to give two products C(C7H12O2) and sodium salt which on nentralisation gave D(C2H,20). Con oxidation also gave 'D'. On the basis of the reactions give above answer the following questions.
The structure of 'B' is likely to be
View SolutionCompounds A(C9H16) decolourises Br, in CCI4 A reacts alkaline KMnO4 to give a diol. A on ozonolysis gave B(C7H12O) and acetaldehyde. B reacts in presence of sodium hydroxide to give two products C(C7H12O2) and sodium salt which on nentralisation gave D(C2H,20). Con oxidation also gave 'D'. On the basis of the reactions give above answer the following questions.
The structure of 'D' is likely to be
View SolutionCompound A(C6H13NO) liberates hydrogen on reaction with metallic sodium. A on reaction with NaNO2 and HCl was found to give three products B(C6H10O),C(C6H10O) and D(C6H12O2) a vicinal. Both B & C react with 2,4 – DNP to give coloured product. C reduces Fehling's solution, but not B. B on reduction with HI & Red P gave cyclohexane while C gave methylcyclophentane. D on reduction with HI & Red P also gave cyclohexane.
Study the reaction give above carefully and answer the following questions.
Compound 'B' is
View SolutionCompound A(C6H13NO) liberates hydrogen on reaction with metallic sodium. A on reaction with NaNO2 and HCl was found to give three products B(C6H10O),C(C6H10O) and D(C6H12O2) a vicinal. Both B & C react with 2,4 – DNP to give coloured product. C reduces Fehling's solution, but not B. B on reduction with HI & Red P gave cyclohexane while C gave methylcyclophentane. Don reduction with HI & Red P also gave cyclohexane.
Study the reaction give above carefully and answer the following questions.
Compound 'C' is
View SolutionCompound A(C6H13NO) liberates hydrogen on reaction with metallic sodium. A on reaction with NaNO2 and HCl was found to give three products B(C6H10O),C(C6H10O) and D(C6H12O2) a vicinal. Both B & C react with 2,4 – DNP to give coloured product. C reduces Fehling's solution, but not B. B on reduction with HI & Red P gave cyclohexane while C gave methylcyclophentane. Don reduction with HI & Red P also gave cyclohexane.
Study the reaction give above carefully and answer the following questions.
The structure of Compound 'A' is
View Solution
