For SO2Cl2(g)→SO2(g)+Cl2(g) , Pressure of SO2Cl2 changed from 5 atm to 4 atm in 10 min. Then, pressure of SO2Cl2 at the end of 30 minutes will be (in atm)Solution in Telugu
2.56
3.56
4.56
5.56
The correct Answer is:A
Step by step video, text & image solution for For SO_2Cl_2(g) to SO_2(g) +Cl_2(g) , Pressure of SO_2Cl_2 changed from 5 atm to 4 atm in 10 min. Then, pressure of SO_2Cl_2 at the end of 30 minutes will be (in atm) by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
For SO_2Cl_2(g)to SO_2(g) + Cl_2(g) Pressures of SO_2Cl_2 at t = 0 and t =20 minutes respectively are 700 mm & 350 mm. When log (P_0//p) is plotted against time (t), slope equals to
View Solutionकिसी निश्चित आयतन पर SO2Cl2 के प्रथम कोटि के तापीय विघटन के दौरान निम्न डाटा प्राप्त किया गया।
SO2Cl2(g)→SO2(g)+Cl2(g)
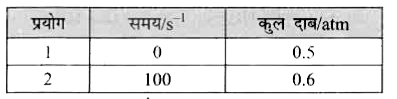
अभिक्रिया की दर क्या है जब कुल दाब 0.65 atm हो?
View SolutionThe volume of the reaction vessel containing an equilibrium mixture in the reaction , SO_2Cl_2(g) hArr SO_2(g) + Cl_2(g) is increased. When equilibrium is reestablished:
View SolutionA reaction, SO2Cl2→SO2+Cl2 is first order reaction with half life period 3.15×104s at 320∘C. What percentage of SO2Cl2 would be decomposed on heating at 320∘C for 90 minutes?
View SolutionA reaction SO_2Cl_2 rarr SO_2 + Cl_2 is first order reaction with half life period 3.15xx10^-4s at 320^@C . What percentage of SO_2Cl_2 would be decomposed on heating at 320^@C for 90 minutes?
View SolutionFor the equilibrium SO_2Cl_2(g) iff SO_2{g) + Cl_2(g) , what is the temperature at which (K_p(atm))/(K_c(M)) = 3 ?
View SolutionThe Reaction SO2Cl2→SO2+Cl2, is a first order reaction with k=2.2×10−5s−1 at 320∘C. Calculate the percentage of SO2Cl2 that is decomposed on heating this gas for 30 minutes.
View SolutionThe following data were obtained during the first thermal decomposition of SO_2Cl_2 at a constant volume, SO_2Cl_2(g) to SO_2 (g)+Cl_2 (g) Experiment Time/s Total pressure/ atm 1 0 0.5 2 100 0.6 Calculate the rate of the reaction when total pressure is 0.65 atm.
View Solution
