When NH3 is treated with HCl, H-N-H bond angle
Increases
Decreases
Remains same
Depends upon temperature
The correct Answer is:A
Step by step video, text & image solution for When NH_(3) is treated with HCl, H-N-H bond angle by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:AAKASH INSTITUTEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:Assignment Section - C Objective Type Questions(More than one options are correct )
Explore 13 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:AAKASH INSTITUTEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:Assignment Section - D (Linked Comprehension Type Questions)
Explore 11 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTURE Book:AAKASH INSTITUTEChapter:CHEMICAL BONDING AND MOLECULAR STRUCTURE Exercise:Assignment Section -A Objective Type Questions (One option is correct)
Explore 60 Videos
- BIOMOLECULESBook:AAKASH INSTITUTEChapter:BIOMOLECULESExercise:EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions
Explore 5 Videos
- CHEMICAL KINETICSBook:AAKASH INSTITUTEChapter:CHEMICAL KINETICSExercise:ASSIGNMENT (SECTION D : Assertion - Reason Type Questions)
Explore 15 Videos
Similar Questions
CO2 has same geometry as .
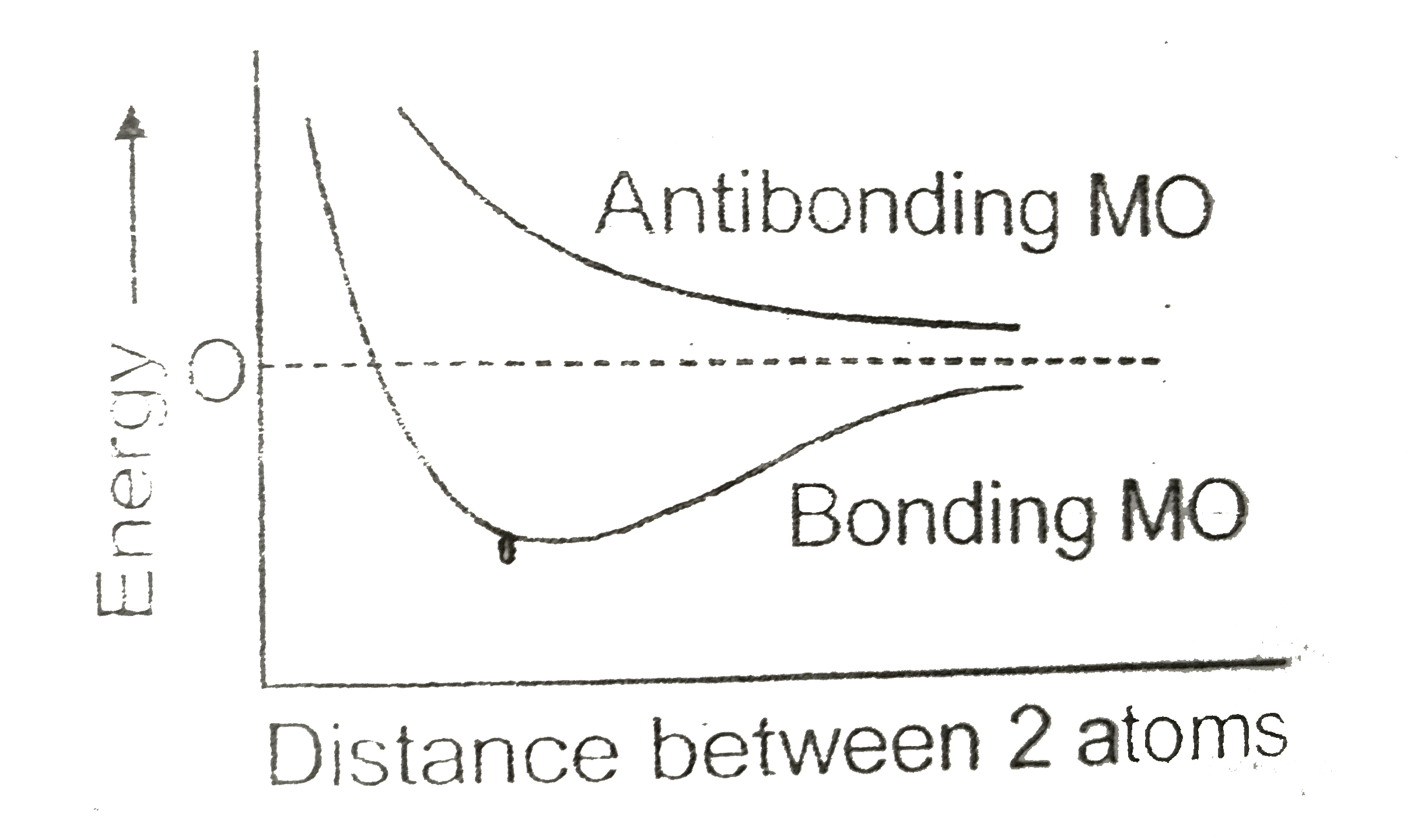
View SolutionMolecular orbital (MOT) the atoms in a molecule are supposed to loose their individual control over the electrons . The nuclei of the bonded atoms are considered to be present at equilibrium in inter-nuclear positions . The orbitals where the probability of finding electrons is maximum are called molecular orbitals extending over 2 or more nuclei when a pair of atomic orbitals combine and give rise to a pair of molecular MO's in internuclear region but decreased for antibonding MO's . Shielding of the nuclei by-increased electron density in bonding MO's reduces inter nuclear repulsion & thus stabilizes the molecule whereas lower electron density in antibonding MO's increases the repulsion and destabilises the system .
Which of the following is the correct order of bond strength of given species ?
View SolutionWhen two atoms combine to form a molecule .
View SolutionA : Glucose and Mannose are Anomers.
R : Configuration at 1st carbon differ in Gluco& and Mannose.
View SolutionA : Rate of reaction depends upon the concentration of the reactants.
R : The order of reaction can be negative with respect to substance present in the reaction.
View SolutionHeat of formation of 2mol of NH3(g) is =90kJ, bond energies of H−H and N−H bonds are 435kJ and 390kJmol−1, respectively. The value of the bond enegry of N≡N will be
View SolutionIf bond enthalpies Of N-=N,H-Hand N-H bonds are x_(1),x_(2)and x_(3) respectively , DeltaH_(f)^(@) for NH_(3) will be :
View SolutionWhy is the H-O-H bond angle in H_(2)O smaller than H-N-H bond angle in NH_(3) ?
View SolutionAssertion (A) : Though the central atom of both NH_(3) and H_(2)O molecules are sp^(3) hybridised , yet H-N-H bond angle is greater than that of H-O-H.
View SolutionArrange the following molecules/ions in order of decreasing H-N-H bond angle and explain the order. NH_(3),NH_(4)^(+),NH_(2)^(-) .
View SolutionThe nitrogen atom in NH3,NH−2 and NH+4 are all surrounded by eight electrons. When these species are arranged in increasing order of H−N−H bond angle, correct order is:
View SolutionWhy the H – P – H bond angle in PH_(3) is less than H – N – H bond angle in NH_(3) ?
View SolutionNH3 में H−N−H बन्ध कोण का मान, AsH3 में H−As−H बन्ध कोण से अधिक होता है |
View SolutionH−N−H bond angle in NH3 is ……………. .
View SolutionWhat is the bond angle in the molecules of ammonia ?
View Solution
