In a CCP structure, calculate the minimum distance two tetrahedral voids.
Video Solution
Text Solution
Projection of a tetrahedral void at adjacent edge is at a distance a4 from respective corner.
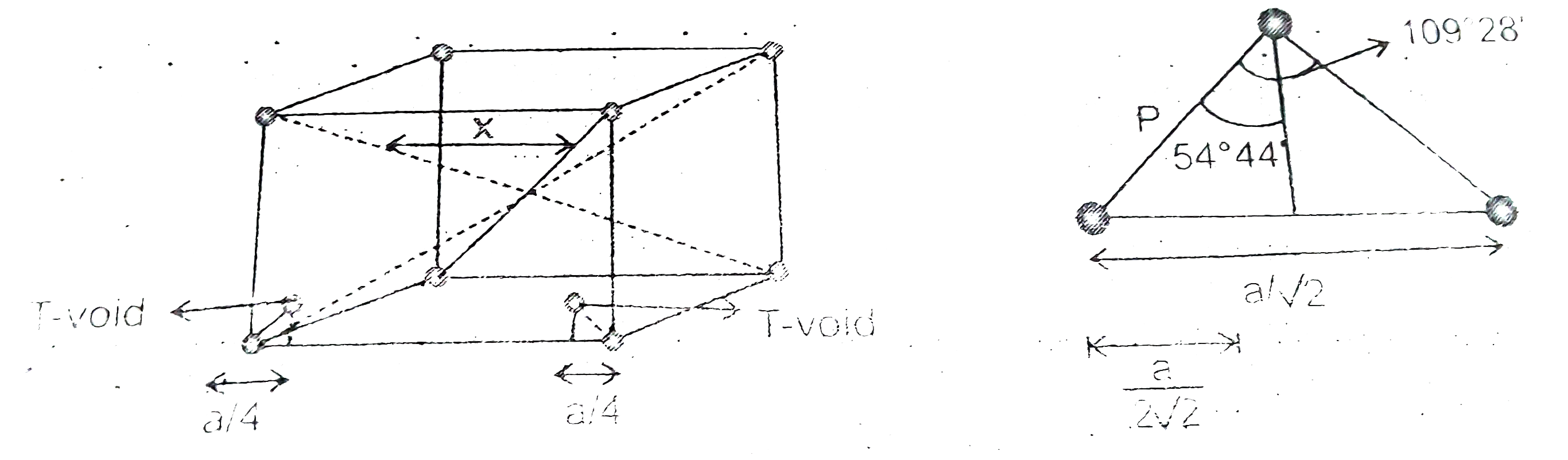
Minimum distance , x=a−(a4+a4)⇒x=a2
|
Similar Questions
If the crystallises in zinc blende structure with I− ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions ?
View SolutionIf the crystallises in zinc blende structure with I− ions at lattice points. What fraction of tetrahedral voids is occupied by Ag+ ions ?
View Solution
Knowledge Check
Which of the following fcc structure contain cations in alternate tetrahedral voids?
ANaClBZnSCNa2ODCaF2Which of the following fcc structure contain cations in alternate tetrahedral voids?
ANaClBZnSCNa2ODCaF2Which of the following FCC structure contains cations in alternate tetrahedral voids?
ANaClBZnSCNa2ODCaF2
Which of the following fc c structure contains cations in alternate tetrahedral voids ?
View SolutionMgFe2O4 has spinel structure , then the percentge of tetrahedral voids and the octahedral voids occupied are respectively
View SolutionWhich of the following fcc structure contain cations in alternate tetrahedral voids?
View SolutionIn a F.C.C arrangement edge length of unit cell is a, which of the following is correct distance between two nearest tetrahedral voids?
View Solutioncoordination number of Tetrahedral void is
View Solution

