Which of the following is a colligative propertySolution in Telugu
vapour pressure of a liquid
boiling point
freezing point
relative lowering of vapour pressure of a solution
The correct Answer is:D
Step by step video, text & image solution for Which of the following is a colligative property by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -2
Explore 110 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -3 (PREVIOUS NEEET/AIPMT )
Explore 24 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Subjective Exercise-3 (Problems)
Explore 7 Videos
- SOLIDS STATEBook:AAKASH SERIESChapter:SOLIDS STATEExercise:PRACTICE EXERCISE
Explore 63 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)
Explore 2 Videos
Similar Questions
The number of Glucose molecules present in 10 ml of decimolar solution is
View SolutionA solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressures of pure hydrocarbons at 20∘C are 440 mm Hg for pentane and 120 mm Hg for hexane. The mole fraction of pentane in vapour phase woule be
View SolutionOne liter aqueous solutions contains 2×10−2 kg glucose at 25∘C . Calculate the osmotic pressure of the solution.
View SolutionWhich of the following is considered as true amorphous solid
View SolutionAt a particular temperature and pressure for a real gas Van der Waal's equation can be written as:
(P+aV2m)(Vm−b)=RT
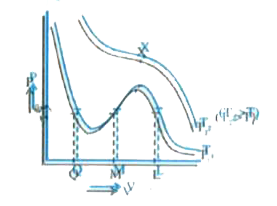
where Vm is molar volume of gas. This is cubic equation in the variable Vm and therefore for any single value of P & T there should be 3 values of Vm. Which are shown in graph as Q, M and L.
As temperature is made to increase at a certain higher temperature the three values of Vm becomes identical. The temperature, pressure & molar volume at point X are called Tc, Pc & Vc for real gas. The compressibility factor in terms of Pc, Vc and T is called Zc.
The expression of Van dcr Waal's constant 'a' can be given as
View SolutionWhich of the following is not a colligative property?
View SolutionWhich of the following is not a colligative property?
View SolutionWhich of the following is 'not' a colligative property ?
View SolutionWhich of the following is 'not' a colligative property?
View SolutionWhich one of the following is not a colligative property?
View SolutionWhich is not a colligative property ?
View SolutionWhich of the following is not a colligative property?
View SolutionWhich of the following is not a colligative property ?
View SolutionWhich of the following is not the colligative property ?
View SolutionWhich of the following is not a colligative property ?
View SolutionWhich of the following is not a colligative property?
View Solution
