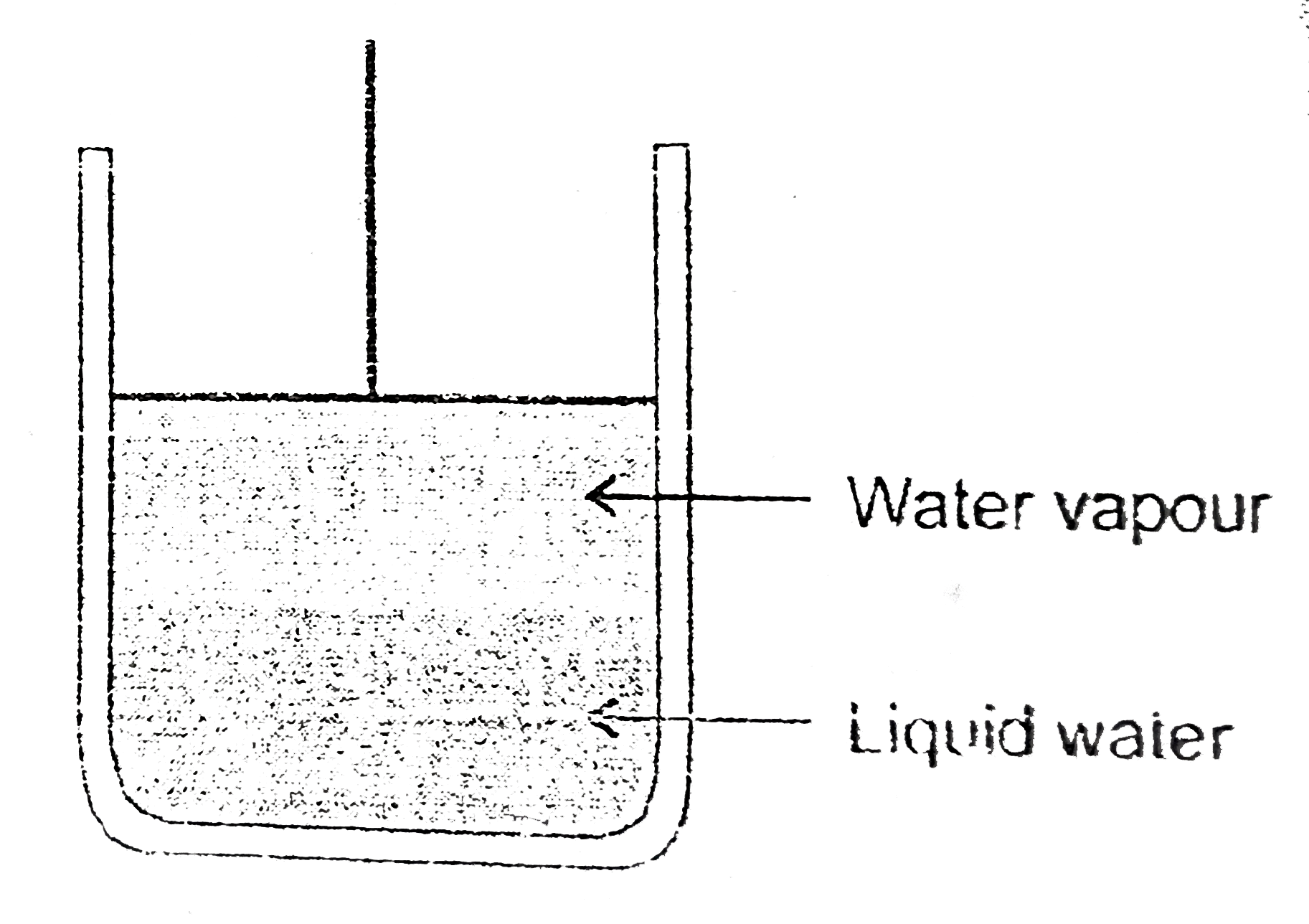
The vapour pressure of water at 20∘C is 17.54mm Hg. then the vapour pressure of the water in the apparatus shown after the piston is lowered, decreasing the volume of the gas above the liquid to one half of its initial volume (temp. constant) isSolution in Telugu
8.77 mm Hg
17.54 mm Hg
35.08 mm Hg
between 8.77 and 17.54 mmHg
The correct Answer is:B
Step by step video, text & image solution for The vapour pressure of water at 20^@C is 17.54mm Hg. then the vapour pressure of the water in the apparatus shown after the piston is lowered, decreasing the volume of the gas above the liquid to one half of its initial volume (temp. constant) is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The vapour pressure of water is 17.5 mm at 20^(@)C temp. what is the vapour pressure of solution 20^(@)C prepared by dissolving 18 gram glucose in to 178.2 gram water at 20^(@)C temp. ?
View SolutionAir inside a closed container is saturated with water vapour . The air pressure is p and the saturated vapour pressure of water is →p. If the mixture is compressed to one half of its volume by maintaining temperature constant, the pressure becomes
View SolutionA cylinder fitted with a movable piston contains liquid water in equiliberium with water vapour at 25^o C . Which operation result in a decrease in the equiliberium vapour pressure ?
View SolutionA vessel has nitrogen gas and water vapour in equilibrium with liquid water at a total pressure of 1 atm. The partial pressure of water vapours is 0.3 atm. The volume of this vessel is reduced to one third of the original volume, at the same temperature, then total pressure of the system is: (Neglect volume occupied by liquid water)
View SolutionThe vapour pressure of water at 20∘C is 17.54mmHg. What will be the vapour pressure of the water in the apparatus shown after the piston is lowered, decreasing the volume of the gas above the liquid to one half of its initial volume (assume temperature is constant).
View Solution2 g of a gas collected over water at 20^(@)C and under a pressure of 770mm Hg occupied 800ml. Calculate the volume of dry gas at S.T.P. condition. Vapour pressure of water at 20^(@)C is 15mm Hg.
View SolutionThe vapour pressure of water at 20^(@)C is 17.54mm . When 20g of non - ionic substance is dissolved in 100g of water, the vapour pressure is lowered by 0.30mm . What is the molecular mass of the substance ?
View Solution

