The molal freezing point constant for water is 1.86 K.kg mole−1. The freezing point of 0.1m NaCl solution is
−1.86∘C
−0.372∘C
−0.186∘C
0.372∘C
The correct Answer is:B
Topper's Solved these Questions
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -3 (PREVIOUS NEEET/AIPMT )
Explore 24 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -4
Explore 50 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -1
Explore 115 Videos
- SOLIDS STATEBook:AAKASH SERIESChapter:SOLIDS STATEExercise:PRACTICE EXERCISE
Explore 63 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)
Explore 2 Videos
Similar Questions
A solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressures of pure hydrocarbons at 20∘C are 440 mm Hg for pentane and 120 mm Hg for hexane. The mole fraction of pentane in vapour phase woule be
View SolutionAssertion : Molarity of a solution decreases with an increase of temperature
Reason : As temperature increases volume of the solution increases.
View SolutionIf air is taken as a binary solution, the solvent is
View SolutionWhich of the following is considered as true amorphous solid
View SolutionAt a particular temperature and pressure for a real gas Van der Waal's equation can be written as:
(P+aV2m)(Vm−b)=RT
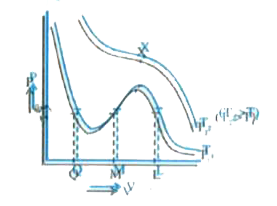
where Vm is molar volume of gas. This is cubic equation in the variable Vm and therefore for any single value of P & T there should be 3 values of Vm. Which are shown in graph as Q, M and L.
As temperature is made to increase at a certain higher temperature the three values of Vm becomes identical. The temperature, pressure & molar volume at point X are called Tc, Pc & Vc for real gas. The compressibility factor in terms of Pc, Vc and T is called Zc.
The expression of Van dcr Waal's constant 'a' can be given as
View SolutionThe freezing point of one molal NaClsolution assuming NaClto be 100%dissociated in water is (molal depression constant=1.86)
View SolutionThe freezing point of one molal NaClsolution assuming NaClto be 100%dissociated in water is (molal depression constant=1.86)
View SolutionMolal depression constant for water is 1.86^(@)C kg/mole. The freezing point of a 0.05 molal solution of a non-electrolyte in water is
View SolutionWhat will be the freezing point of a 0.5 m KCl solution ? The molal freezing point constant of water is 1.86∘Cm−1 .
View SolutionThe molal freezing point constant of water is 1.86^(@)C//M . Therefore the freezing point of 0.1 M NaCl solution in water is expected to be
View SolutionThe molal freezing point constant for water is 1.86^(@)C//m . Therefore, the freezing point of 0.1 M NaCl solution in water is expected to be:
View SolutionThe molal freezing point constant for water is 1.86∘C/m. Therefore, the freezing point of 0.1 M NaCl solution in water is expected to be:
View SolutionThe molal freezing point constant for water is 1.86^(@)C//m . Therefore, the freezing point of 0.1 M NaCl solution in water is expected to be
View Solutionएक मोलल सोडियम क्लोराइड विलयन में NaCI को यदि 100% वियोजित मानते हुए विलयन का हिमांक निकालें तो हिमांक होगा Kf = 1.86
View Solution0.5 m KCI के विलयन का हिमांक क्या होगा? जल का मोलल हिमांक स्थिरांक 1.86∘Cm−1 है।
View Solution

