If molality of the dilute solution is doubled, the value of molal depression constant (Kf) will beSolution in Telugu
unchanged
doubled
halved
tripled
The correct Answer is:A
Step by step video, text & image solution for If molality of the dilute solution is doubled, the value of molal depression constant (K_f) will be by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -4
Explore 50 Videos
- SOLUTIONS Book:AAKASH SERIESChapter:SOLUTIONS Exercise:Objective Exercise -2
Explore 110 Videos
- SOLIDS STATEBook:AAKASH SERIESChapter:SOLIDS STATEExercise:PRACTICE EXERCISE
Explore 63 Videos
- STATES OF MATTER Book:AAKASH SERIESChapter:STATES OF MATTER Exercise:ADDITIONAL PRACTICE EXERCISE -PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- IV)
Explore 2 Videos
Similar Questions
Assertion : Molarity of a solution decreases with an increase of temperature
Reason : As temperature increases volume of the solution increases.
View SolutionThe number of Glucose molecules present in 10 ml of decimolar solution is
View SolutionWhich of the following is considered as true amorphous solid
View SolutionAt a particular temperature and pressure for a real gas Van der Waal's equation can be written as:
(P+aV2m)(Vm−b)=RT
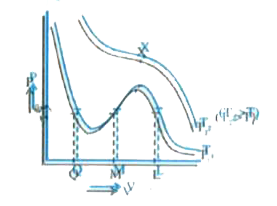
where Vm is molar volume of gas. This is cubic equation in the variable Vm and therefore for any single value of P & T there should be 3 values of Vm. Which are shown in graph as Q, M and L.
As temperature is made to increase at a certain higher temperature the three values of Vm becomes identical. The temperature, pressure & molar volume at point X are called Tc, Pc & Vc for real gas. The compressibility factor in terms of Pc, Vc and T is called Zc.
The expression of Van dcr Waal's constant 'a' can be given as
View SolutionThe molal depression constant for water is 1.86^(@)C . The freezing point of a 0.05-molal solution of a non-electrolyte in water is
View SolutionThe molal depression constant depends upon
View SolutionMolal depression constant for water is 1.86^(@)C kg/mole. The freezing point of a 0.05 molal solution of a non-electrolyte in water is
View SolutionThe molal elevation/depression constant depends on
View SolutionIf molality of the dilute solution is doubled, the value of the molal depression constant (K_(f)) will be
View SolutionGive the unit for molal depression constant.
View Solutionजल का मोलल अवनमन स्थिरांक 1.86∘CKg/mol है। एक विद्युत अनपघट्य के 0.05 मोलल जलीय विलयन का हिमांक होगा
View Solutionजल का मोलल अवनमन स्थिरांक 1.86 है। एक विद्युत अनअपघटय के 0.05 मोलल जलीय विलयन का हिमांक होगा
View SolutionWhat is molal depression constant ?
View SolutionMolal depression constant is given by the expression:
View SolutionMolal depression constant for water is 1.86 K.Kg. "mole"^(-1) . The freezing point of a 0.05 molal solution of a non electrolyte in water is
View SolutionMolal depression constant for water is 1.86^@C. The freezing point of a 0.05 molal solution of a non electrolyte in water is:
View Solution
