Which is more basic among benzyl amine and aniline?Solution in Telugu
Video Solution
Text Solution
Benzyl amine is a stronger base than aniline.
In aniline, lone pair is in conjugation with benzene ring the not in free state.
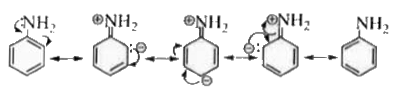
In benzyl amine, the lone pair is in side chain and not in conjugation with the benzene ring. Hence it is in free state and can donate the electron pair easily.
|
Similar Questions
How mnay of the following amines are more basic than aniline?
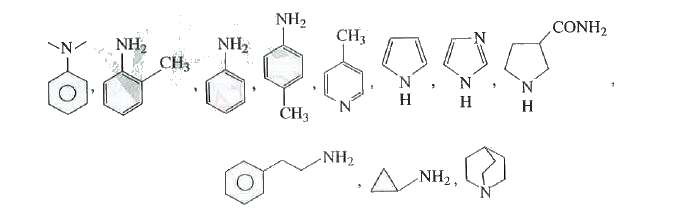
View SolutionArrange the following in the decreasing order of their basic strength: (i) Aniline, ortho, meta and para toluidines (ii) Benzyl amine and aniline (iii) Aniline and cyclohexylamine and (iv) Aniline, N-methylaniline and N, N-dimethylaniline
View SolutionName a test by which you can distinguish between aniline and benzyl amine.
View SolutionWhy methyl amine is more basic than aniline ?
View SolutionAliphatic primary amine is more basic than aniline. Explain with reason.
View SolutionBasic Nature OF Amines || Aniline Derivatives
View SolutionAniline is a stronger base than benzyl amine.
View Solution
