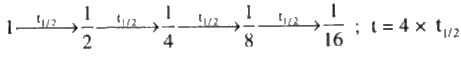Half life period of a radioactive substance is 1600 minutes . What fraction of the substance will remain after 6400 minutes :
A
1/16
B
1/4
C
1/8
D
1/2
Text Solution
The correct Answer is:A
|
Topper's Solved these Questions
- NUCLEAR CHEMISTRY BOOK - AAKASH SERIESCHAPTER - NUCLEAR CHEMISTRY EXERCISE - LECTURE SHEET (LEVEL- II) (EXERCISE - II) (Linked Comprehension type questions)
9 Videos
- NUCLEAR CHEMISTRY BOOK - AAKASH SERIESCHAPTER - NUCLEAR CHEMISTRY EXERCISE - LECTURE SHEET (LEVEL- II) (EXERCISE - III) (Match the following questions )
3 Videos
- NOBLE GASESBOOK - AAKASH SERIESCHAPTER - NOBLE GASESEXERCISE - PRACTICE SHEET - 2 (Single more than one option questions)
5 Videos
- PERIODIC TABLEBOOK - AAKASH SERIESCHAPTER - PERIODIC TABLEEXERCISE - QUESTIONS FOR DESCRIPTIVE ANSWERS
37 Videos
Similar Questions
Knowledge Check
The half life period of radio active isotope is 5 minutes. The fraction of isotope, that will be remaining after 30 minutes is
A1/16B1/36C1/64D1/32The half life period of a radioactive substance is 10 year. The amount of the substance decayed after 40 years would be
A0.25B0.125C0.9375D0.0625The half-life period of a first order reaction is 35 minutes. What fraction of the reactant remains after 75 minutes?
A4.415B0.226C5.263D0.155
The half-life period of a radioactive element is 1 h. After 3 h, what fraction of it will remain?
View SolutionThe half life period of a radioactive element is 90 minutes. What percentage of radioactive element will be present after 6 hours of decomposition ?
View SolutionHalf life of a radioactive substance is 6 minute. If its initial amount is 32g, then amount present after 18 minute is
View SolutionThe half-life period of radium is 1600 years. The fraction of a sample of radium that would remain after 6400 years is.
View SolutionA radioactive substance has t1/2 60 minutes. After 3 hrs, what precentage of radioactive substance will remain
View Solution