A compound is formed by elements A and B. This crystallizes in the cubic structure where the A atoms are at the corners of the cube and B atoms are at the body centres. The simplest formula of the compound is Solution in Telugu
AB
A2B
AB2
AB6
The correct Answer is:A
Step by step video, text & image solution for A compound is formed by elements A and B. This crystallizes in the cubic structure where the A atoms are at the corners of the cube and B atoms are at the body centres. The simplest formula of the compound is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLID STATEBook:AAKASH SERIESChapter:SOLID STATEExercise:LEVEL - II LECTURE SHEET (EXERCISE - I)
Explore 31 Videos
- SOLID STATEBook:AAKASH SERIESChapter:SOLID STATEExercise:LEVEL - II LECTURE SHEET (EXERCISE - II)
Explore 6 Videos
- SOLID STATEBook:AAKASH SERIESChapter:SOLID STATEExercise:LEVEL - I (EXERCISE - I)
Explore 68 Videos
- REVISION EXERCISE Book:AAKASH SERIESChapter:REVISION EXERCISE Exercise:COMPLEX COMPOUNDS
Explore 47 Videos
- SOLIDS STATEBook:AAKASH SERIESChapter:SOLIDS STATEExercise:PRACTICE EXERCISE
Explore 63 Videos
Similar Questions
If 'a' stands for the edge length of the cubic systems: simple cubic, body centered cubic and face centred cubic, then the ratio of radii of the spheres in these systems will be respectively. 2.
View Solution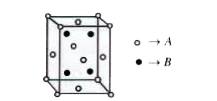
If the molar mass AB is 100gmol−1 and 'a' is edge length then the density of the crystal will be
View SolutionWhich of the following is amorphous in nature?
View SolutionIn which of the following complexes the metal ion is in zero oxidation state ?
View SolutionWhich of the following is considered as true amorphous solid
View SolutionA compound formed by elements A and B crystallizes in cubic structure, where A atoms are at the corners of a cube and B atoms are at the face - centre. What is the formula of the compound ?
View SolutionA compound is formed by elements A and B. This crystallises in the cubic structure when atoms A are at the corners of the cube and atoms B are at the centre of the body.The simplest formula of the compound is
View SolutionA compound is formed by elements A and B . This crystallizes in the cubic structure where the A atoms are at the corners of the cube can B atoms are at the body centers. The simplest formula of the compound is
View SolutionA compound is formed by elements A and B. This crystallizes in the cubic structure when atoms are the corners of the cube and atoms B are at the centre of the body. The simplest formula of the compounds is
View SolutionA compound formed by elements A and B crystallizes in cubic structure, where A atoms are at the corners of a cube and B atoms are at the face − centre. What is the formula of the compound ?
View SolutionA compound formed by element A and B crystallize in the cubic structure where A atoms are at the corners of a cube and B atoms are at the centre of the body. The formula of the compounds is
View SolutionA compound is formed by elements A and B . This crystallises in the cubic structure where the A atoms are the corners of the cube and B atoms are at the body centres . The simplest formula of the compound is
View SolutionA compound is formed by elements A and B. This crystallizes in the cubic structure where the A atoms are at the corners of the cube and B atoms are at the body centres. The simplest formulta of the compound is :
View SolutionA compound is formed by elements A and B. This crystallizes in the cubic structure where the A atoms are at the corners of the cube and B atoms are at the body centres. The simplest formula of the compound is
View Solutionएक यौगिक A तथा B तत्वों से बनता है। यह घनीय संरचना में क्रिस्टलीकृत होता है जिसमें परमाणु A घन के कोनों पर तथा परमाणु B निकाय के केन्द्र पर स्थित होता है तो यौगिक का सरलतम सूत्र होगा
View Solution
