To measure the pressure exerted by a trapped gas A, an open ended mercury manometer is used. After sparking the gas A, it dissociates as, A(g) → B(g) + 3C(g). If the pressure of A decreases to 0.9 atm, then (assume temperature to be constant, 300K)Solution in Telugu
total pressure increased to 1.3 atm
total pressure increased by 0.3 atm
difference in mercury level is 228 mm
Total pressure increased by 0.3 cm of Hg
A(g)→B(g)+3C(g)1001−xx3x
Similar Questions
(i) 0.24 g of a gas dissolves in 1 L of water at 1.5 atm pressure. Calculate the amount of dissolved gas when the pressure is raised to 6.0 atm at constant temperature. (i) What is a vapour pressure of liquid?
View SolutionTo measure the pressure exerted by a trapped gas A, an open ended mercury manometer is used. After sparking the gas A, it dissociates as, A(g) to B(g) + 3C(g). If the pressure of A decreases to 0.9 atm, then (assume temperature to be constant, 300K)
View SolutionAt 273 K temperature and 9 atm pressure, the compresibility for a gas is 0.9. The volume of 1 milli- moles of gas at this temperature and pressure is
View Solutionचित्र में दर्शाए गए खुले सिरे वाले मरकरी मैनोमीटर का उपयोग फंसी हुई गैस द्वारा उत्पन्न दाब मापने में किया जाता है प्रारम्भ में चित्र में दर्शाये गये मैनोमीटर P=76 सेमी के दोनों स्तम्भों में मरकरी तल में कोई अंतर नहीं है। चमक के बाद 'A' का वियोजन निम्न अभिक्रिया के गैसA अनुसार होता है A(lg)→Blg)+3Cl(g) यदि गैस "A" के दाब को 0.9atm. तक घटाते है तब (कल्पित तापक्रम स्थिर तथा 300 K होता है)
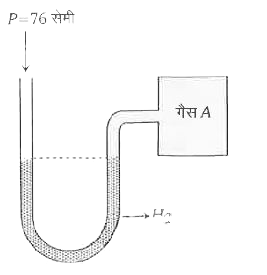
View SolutionA open ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Initially manometer shows no difference in mercury level in both columns as shown in diagram. After sparking 'A' dissociates according to following reaction A(g)rarrB(g)+3C(g) If pressure of Gas "A" dissociates to 0.9 atm, then (Assume temperature to be constant and is 300 K)
View Solutionप्रारम्भ में एक पात्र में 1 g गैस A एक नियत ताप पर 4 atm दाब रखती है, यदि समान ताप पर उसी पात्र में 2 g गैस B मिला दी जाए तो दाब 6 atm हो जाता है तो A तथा B के अणुभार का अनुपात होगा :
View SolutionFor the reaction PCI5(g)⇔PCl3(g)+Cl2(g) the forward reaction at constant temperature is favoured by
(1) introducing an inert gas at constant volume
(2) introducing chlorine gas at constant volume
(3) introducing an inert gas at constant pressure
(4) decreasing the volume of the container
(5) introducing PCl, at constant pressure
View Solution
