For the adiabatic expansion of an ideal gasSolution in Telugu
PVγ= constant
TVγ−1= constant
TγP1−γ= constant
none of these
The correct Answer is:A, B, C
PVγ= constant
⇒TVγ−1 = constant ⇒TγP1−γ= constant
Topper's Solved these Questions
- CHEMICAL THERMODYNAMICSBook:AAKASH SERIESChapter:CHEMICAL THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) LINKED COMPREHENSION TYPE QUESTIONS)
Explore 5 Videos
- CHEMICAL THERMODYNAMICSBook:AAKASH SERIESChapter:CHEMICAL THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) MATRIX MATCHING TYPE QUESTIONS)
Explore 1 Video
- CHEMICAL THERMODYNAMICSBook:AAKASH SERIESChapter:CHEMICAL THERMODYNAMICSExercise:LECTURE SHEET (EXERCISE-I) (LEVEL - II (ADVANCED) STRAIGHT OBJECTIVE TYPE QUESTIONS)
Explore 5 Videos
- CHEMICAL THERMODYANMICS Book:AAKASH SERIESChapter:CHEMICAL THERMODYANMICS Exercise:Questions For Descriptive Answers
Explore 28 Videos
- ELECTRON MIGRATION EFFECTS Book:AAKASH SERIESChapter:ELECTRON MIGRATION EFFECTS Exercise:QUESTIONS FOR DESCRIPTIVE ANSWERS
Explore 10 Videos
Similar Questions
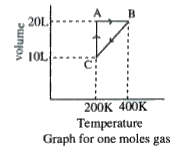
Process A→B represents
View SolutionMatch the following columns
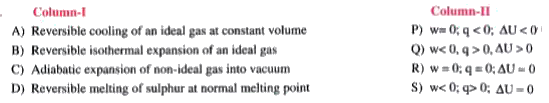
View SolutionWhich has maximum internal energy at 298K?
View SolutionHow does heat of neutralisation value change with the chemical nature of acid and base?
View SolutionHyperconjugation effect is also termed as 'no bond resonance'. Why?
View SolutionFor the adiabatic expansion of an ideal gas:
View SolutionFor the adiabatic expansion of an ideal gas:
View SolutionIn an adiabatic expansion of an ideal gas -
View SolutionFor the adiabatic expansion of an ideal gas:
View SolutionDuring adiabatic expansion of an ideal gas :-
View SolutionIn an adiabatic expansion of an ideal gas
View SolutionFor the adiabatic expansion of an ideal gas
View SolutionIn an adiabatic expansion of an ideal gas
View SolutionIn an adiabatic expansion of an ideal gas
View SolutionIn an adiabatic expansion of an ideal gas.
View Solution
