Covalent bond length of chlorine molecule is 1.98Å. Then covalent radius of chlorine isSolution in Telugu
1.98 Å
1.7 Å
2.05 Å
0.99 Å
The correct Answer is:D
Step by step video, text & image solution for Covalent bond length of chlorine molecule is 1.98Å. Then covalent radius of chlorine is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC CLASSIFICATIONBook:AAKASH SERIESChapter:PERIODIC CLASSIFICATIONExercise:OBJECTIVE EXERCISE - 1 (IONISATION POTENTIAL)
Explore 25 Videos
- PERIODIC CLASSIFICATIONBook:AAKASH SERIESChapter:PERIODIC CLASSIFICATIONExercise:OBJECTIVE EXERCISE - 1 (ELECTRON AFFINITY)
Explore 13 Videos
- PERIODIC CLASSIFICATIONBook:AAKASH SERIESChapter:PERIODIC CLASSIFICATIONExercise:OBJECTIVE EXERCISE - 1 (BLOCK AND TYPES OF ELEMENTS)
Explore 40 Videos
- ISOMERISM Book:AAKASH SERIESChapter:ISOMERISM Exercise:ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)
Explore 10 Videos
- PERIODIC PROPERTIESBook:AAKASH SERIESChapter:PERIODIC PROPERTIESExercise:LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))
Explore 8 Videos
Similar Questions
How many ionisation energies can carbon have ? Electron configuration of carbon
in Ground state1s22s22p1x2p1yin Excited state1s22s12p1x2p1y2p1z
View SolutionElectron affinity is
View SolutionIf Aufbau rule is not followed, K - 19. will be placed in
View SolutionTautomerism is exhibited by X number of compounds among the following. What is X.
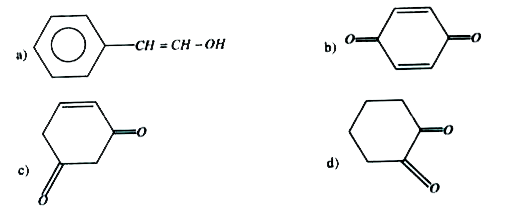
View SolutionWhat is the valency possible to Arsenic with respect to oxygen and hydrogen?
View Solutionvan der Waals radius of chlorine is less than that of covalent radius.
View SolutionCovalent bond length of chlorine molecule is 1.98A^(0) . Covalent radius of chlorine is
View SolutionThe covalent radius of hydrogen is 0.37A0. The bond length in H2 molecule is
View SolutionWhat is covalent radius ? . How would you determine the covalent radius of chlorine atom .
View Solutionनिम्नलिखित को समझाइए -
क्लोरीन की वांडरवाल त्रिज्या सहसंयोजक त्रिज्या से अधिक होती है।
View SolutionCovalent bond length of chlorine molecules is 1.98 A. Covalent radius in ( in A) of chlorine atom is
View SolutionWhat is the maximum covalency of chlorine?
View SolutionThe Max. covalency of chlorine is
View SolutionThe max. covalency of chlorine is
View SolutionCovalency of Chlorine molecule?
View Solution
