Which of the following ionization energy values for Calcium show a sudden increase ? Solution in Telugu
Third
Second
First
Fourth
The correct Answer is:A
Step by step video, text & image solution for Which of the following ionization energy values for Calcium show a sudden increase ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC CLASSIFICATIONBOOK - AAKASH SERIESCHAPTER - PERIODIC CLASSIFICATIONEXERCISE - OBJECTIVE EXERCISE - 1 (ELECTRON AFFINITY)
13 Videos
- PERIODIC CLASSIFICATIONBOOK - AAKASH SERIESCHAPTER - PERIODIC CLASSIFICATIONEXERCISE - OBJECTIVE EXERCISE - 1 (ELECTRONEGATIVITY)
15 Videos
- PERIODIC CLASSIFICATIONBOOK - AAKASH SERIESCHAPTER - PERIODIC CLASSIFICATIONEXERCISE - OBJECTIVE EXERCISE - 1 (ATOMIC AND IONIC RADIUS)
29 Videos
- ISOMERISM BOOK - AAKASH SERIESCHAPTER - ISOMERISM EXERCISE - ADDITIONAL PRACTICE EXERCISE ( PRACTICE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)
10 Videos
- PERIODIC PROPERTIESBOOK - AAKASH SERIESCHAPTER - PERIODIC PROPERTIESEXERCISE - LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))
8 Videos
Similar Questions
Electron affinity is
View SolutionPauling's electronegativity is based on
View Solution(A) : Mg2+ and Al3+ are isolectronic but the magnitude of ionic radius of Al3+ is less than that in Mg2+.
(R ) : The effective nuclear charge on the outermost electrons in Al3+ is greater than that in Mg2+.
View SolutionTautomerism is exhibited by X number of compounds among the following. What is X.
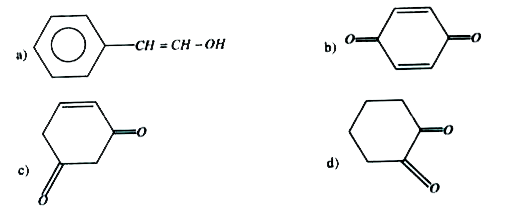
View SolutionWhat is the valency possible to Arsenic with respect to oxygen and hydrogen?
View SolutionWhich of the following ionisation energy valuyes for calcium show a sudden increase?
View SolutionThe ionization potential value of alkali metals which shows abrupt increase is
View Solutionकिसी तत्व कि किस आयनन ऊर्जा के मान में एकदम उछाल दिखता है और क्यों? उदाहरण सहित समझाइए।
View Solutionनिम्न में से कौन आयनन ऊर्जा वृद्धि का सही क्रम है?
View Solutionनिम्नलिखित में से किसके लिए आयनीकरण ऊर्जा का मान उच्चतम है?
View SolutionA sudden large jump between the values of second and third ionisation energies of an element would be associated with the electronic configuration
View SolutionSuccessive ionization energy values increase Why ?
View SolutionAn increase in ______ increases the ionization energy.
View Solutionनिम्न में से सर्वाधिक आयनन ऊर्जा का मान है
View SolutionA sudden long jump between the values of the second and third ionization energies of an element will be associated with which of the following electronic configurations?
View Solution

