Why first ionisation potential of aluminium is less than that of magnesium? Solution in Telugu
Aluminium atom is very large when compared to Mg
Aluminium has a stable electronic configuration
Magnesium has a stable electronic configuration
The electron affinity of Magnesium is positive (energy is absorbed)
The correct Answer is:C
Step by step video, text & image solution for Why first ionisation potential of aluminium is less than that of magnesium? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- PERIODIC TABLEBook:AAKASH SERIESChapter:PERIODIC TABLEExercise:OBJECTIVE EXERCISE -1 (ELECTRON AFFINITY)
Explore 10 Videos
- PERIODIC TABLEBook:AAKASH SERIESChapter:PERIODIC TABLEExercise:OBJECTIVE EXERCISE -1 (ELECTRONEGATIVITY)
Explore 12 Videos
- PERIODIC TABLEBook:AAKASH SERIESChapter:PERIODIC TABLEExercise:OBJECTIVE EXERCISE -1 (ATOMIC AND IONIC RADIUS)
Explore 23 Videos
- PERIODIC PROPERTIESBook:AAKASH SERIESChapter:PERIODIC PROPERTIESExercise:LEVEL-II (TYPE -II) (PRACTICE SHEET -3 (SUBJECTIVE/ ANALYTICAL TYPE QUESTIONS ))
Explore 8 Videos
- PURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATUREBook:AAKASH SERIESChapter:PURIFICATION OF ORGANIC COMPOUNDS AND IUPAC NOMENCLATUREExercise:ADDITIONAL PRCATICE EXERCISE (LEVEL - II (LECTURE SHEET (ADVANCED) INTEGER TYPE QUESTIONS)
Explore 2 Videos
Similar Questions
Electron affinity is
View SolutionThe electron affinity values of elements A,B, C and D are respectively -135, -60, -200 and -348 kJ mol^(-1). The outer electronic configuration of elecment B is
View SolutionWhich of the following is not a periodic property ?
View SolutionWhat is the valency possible to Arsenic with respect to oxygen and hydrogen?
View Solution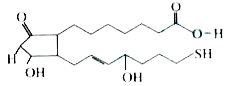
How many types of functional groups are present in given compound
View SolutionAssertion: The first ionisation enthalpy of aluminium is lower than that of magnesium.
Reason : Ionic radius of aluminium is smaller than that of magnesium.
View SolutionAssertion: The first ionisation energy of aluminium is lower than that of magnesium. Reason: The ionic radius of aluminium is smaller than that of magnesium.
View Solutionआप इस तथ्य की व्याख्या किस प्रकार करेंगे की सोडियम की प्रथम आयनन एन्थेल्पी मैग्नीशियम की प्रथम आयनन एन्थेलपी से कम किन्तु इसकी द्वितीय आयनन एन्थेल्पी मैग्नीशियम की द्वितीय आयनन एन्थेल्पी से अधिक है ?
View SolutionThe first ionisation potential of aluminium is smaller than that of magnesium because
View SolutionWhich out of magnesium and aluminium has greater ionisation potential and why?
View SolutionThe first ionisation energies of magnesium and aluminium are respectively given by
View SolutionWhy the first ionisation enthalphy of sodium is lower than that of magnesium while its second ionisation enthlpy is higher than that of magnesium ?
View SolutionWhy is the 1st ionisation potential of B less than that of Be ?
View Solution2nd ionisation potential of Be is greater than the 1st ionisation potential of B but less than 2nd ionisation potential of B - explain .
View SolutionWhy first ionisation potential of aluminium is less than that of magnesium?
View SolutionWhy first ionisation potential of aluminium is less than that of magnesium?
View Solution
