The first (IP1) and the second (IP2) ionisation potential (kJ mol 1) of a few elements designed by roman numerals are shown below
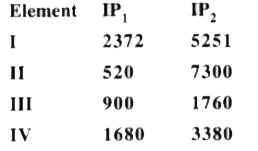
Identify the element which are likely to be a noble gas and a reactive metal respectively Solution in Telugu
I and II
II and III
I and IV
II and IV
The correct Answer is:A
Step by step video, text & image solution for The first (IP_1) and the second (IP_2) ionisation potential (kJ mol 1) of a few elements designed by roman numerals are shown below Identify the element which are likely to be a noble gas and a reactive metal respectively by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
The first (IE1) and second (IE2) ionisation enthalpies (kJ mol−1) of a few elements designated by Roman numerals are shown below.
IE1IF2I23725251III9001760 IE1IE2II5207300IV16803380
Which of the above element is likely to be:
a reactive non-metal
View SolutionThe first (IE_1) and second (IE_2) ionisation enthalpies ("kJ mol"^(-1)) of a few elements designated by Roman numerals are shown below. {:(,,IE_(1),IF_(2)),(,I,2372,5251),(,III,900,1760):} {:(,,IE_(1),IE_(2)),(,II,520,7300),(,IV,1680,3380):} Which of the above element is likely to be: a noble gas
View SolutionThe first (IE1) and the second (IE2) ionization enthalpies (kJ mol−1) of a few elements designated by Roman numerals are shown below:
ElementIE1IE2I23725251II5207300III9001760III9001760IV16803380
Which of the above elements is likely to be a,
reactive metal
View SolutionThe first (IE_1) and the second (IE_2) ionization enthalpies ("kJ mol"^(-1)) of a few elements designated by Roman numerals are shown below: {:(,"Element",IE_(1),IE_(2)),(,I,2372,5251),(,II,520,7300),(,III,900,1760),(,III,900,1760),(,IV,1680,3380):} Which of the above elements is likely to be a, reactive non metal
View SolutionThe first (IE_1) and the second (IE_2) ionization enthalpies ("kJ mol"^(-1)) of a few elements designated by Roman numerals are shown below: {:(,"Element",IE_(1),IE_(2)),(,I,2372,5251),(,II,520,7300),(,III,900,1760),(,III,900,1760),(,IV,1680,3380):} Which of the above elements is likely to be a, noble gas
View SolutionThe first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
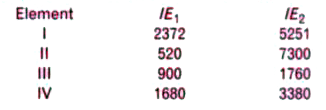
Which of the above elements is likely to be
a reactive metal?
View SolutionThe first (IE1) and the second (IE2) ionisation energies (kJ mol−1) of a few elements are given below :
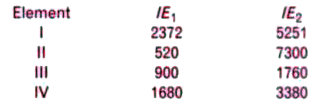
Which of the above elements is likely to be
a reactive non-metal?
View Solution
