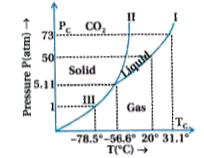
Answer the following questions based on the P - T phase diagram of CO2 :
(a) CO2 at 1 atm pressure and temperature −60∘C is compressed isothermally. Does it go through a liquid phase ?
(b) What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure ?
(c) Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature −65∘C as it is heated up to room temperature at constant pressure.
(d) CO2 is heated to a temperature 70∘C and compressed isothermally. What changes in its properties do you expect to observe ?
 Solution in Gujrati
Solution in Gujrati
(a) No, CO2 is in steam from at 1 atm pressure and −60∘C temperature.
It its isothermal compression is done i.e. increasing pressure with constant temperature then it directly converts into solid form without coming through liquid. (Sublimation)
(b) CO2 is in gaseous state at 4 atm pressure and 27∘C temperature.
If it is clled at constant pressure then it directly converts into gaseous state without coming through liquid. It intersects at curve III.
(c) CO2 is in solid form at 10 atm pressure and −60∘C temp.
If it is heated at constant pressure then first it comes in liquid form and then in gaseous form because it intersects the fusion and vaporization curve.
(d) It is seen from phase diagram of CO2 that the above 70∘C temperature it doesn.t have liquid area. Then also it reacts as an ideal gas with increase in pressure.
Similar Questions
Answer the following questions based on the P-T phase diagram of CO2:
Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature −65∘C as it is heated up to room temperature at constant pressure.
View SolutionAnswer the following questions based on the P-T phase diagram of CO_(2) : CO_(2) at 1 am pressure and temperature - 60^(@) C is compressed isothermally Does it go through a liquid phase ?
View SolutionAnswer the following questions based on the P-T phase diagram of CO2:
What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure ?
View SolutionAnswer the following questions based on the P-T phase diagram of CO2:
Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature −65∘C as it is heated up to room temperature at constant pressure.
View SolutionAnswer the following questions based on the P-T phase diagram of CO2:
CO2 at 1 am pressure and temperature −60∘C is compressed isothermally Does it go through a liquid phase ?
View SolutionAnswer the following questions based on the P-T phase diagram of CO_(2) : What happens when CO_(2) at 4 atm pressure is cooled from room temperature at constant pressure ?
View SolutionAnswer the following questions based on the P-T phase diagram of CO_(2) : Describe qualitatively the changes in a given mass of solid CO_(2) at 10 atm pressure and temperature - 65 ^(@) C as it is heated up to room temperature at constant pressure.
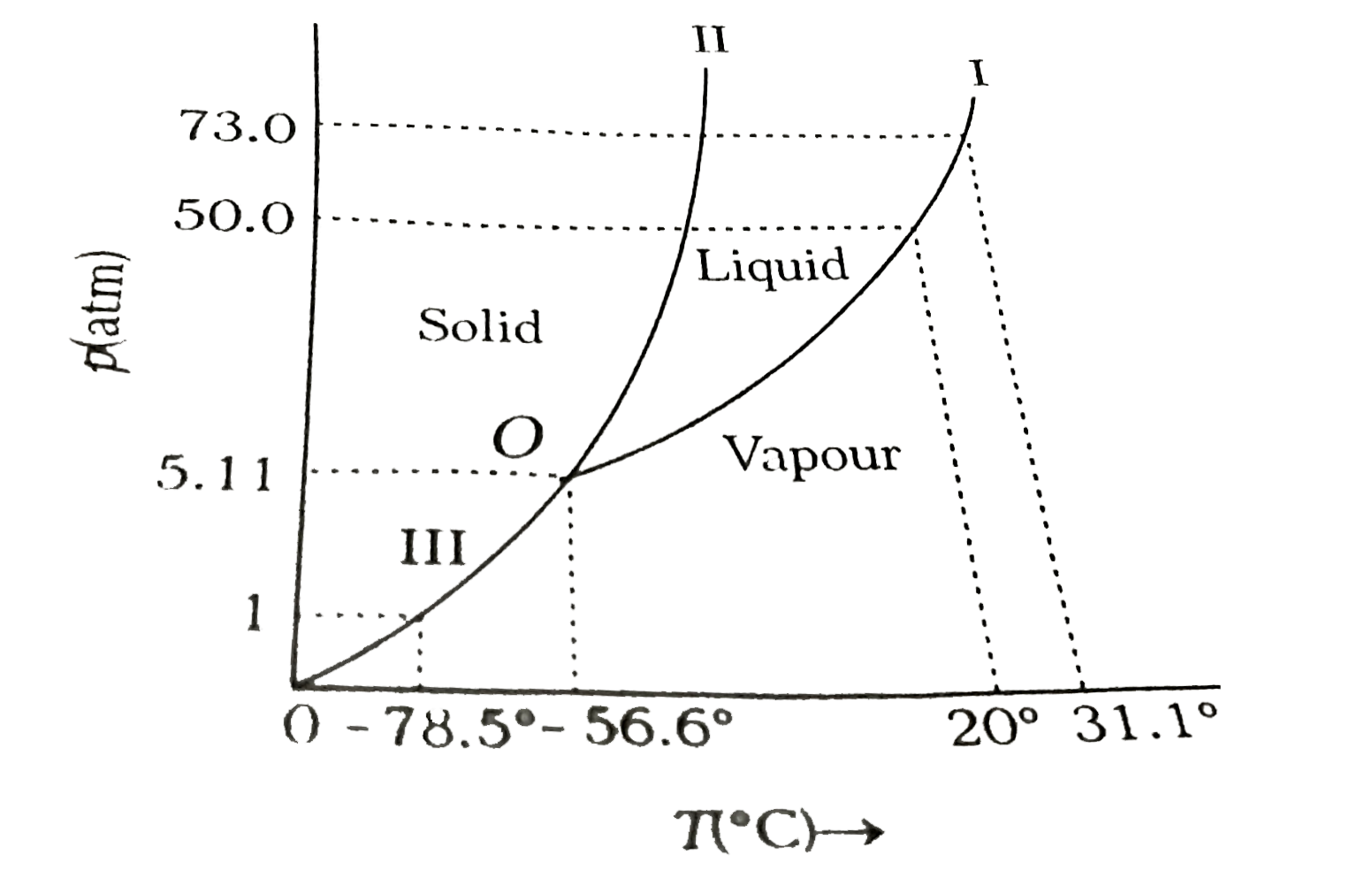
View SolutionAnswer the following questions based on the P – T phase diagram of CO2:
(a) CO2 at 1 atm pressure and temperature–60∘C is compressed isothermally. Does it go through a liquid phase ?
(b) What happens when CO2 at 4 atm pressure is cooled from room temperature at constant pressure ?
(c) Describe qualitatively the changes in a given mass of solid CO2 at 10 atm pressure and temperature –65∘C as it is heated up to room temperature at constant pressure.
(d) CO2 is heated to a temperature 70∘C and compressed isothermally. What changes in its properties do you expect to observe ?
View Solution

