Explain cell, emf and internal resistance. Derive relation between potential difference emf and internal resistance. Solution in Gujrati
⇒ As shown in figure in a glass vessel electrolyte is field in.
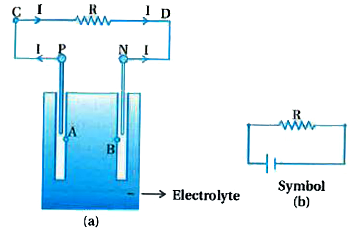
⇒ In this electrolyte tw" electrode are partially dipped. One electrode is positive electrode (P) and second electrolyte is negative (N) .
⇒ Electrodes dipped in electronde exchange electric charge in form of positive and negative ion produced as result of positive and negative ion produced as a result of chaemical reaction.
⇒ Potential difference between electrode A and electrolyte is (V+V+>0) and potential difference between electrolyte and negative electrode is V−V−<0.
⇒ When current is not flowing in the circuit potential drift between two point P and N.
V+−(V−)
= V++V−
⇒ Potential difference between V+andV− is called electromotive force (emf) of the cell, it is denoted by ε.
ε=V++V−>0 .... (1)
⇒ Definition of emf of cell : " When unit positive charge moves from negative to positive terminal of cell due to non electric force then energy gained by charge is called emf of cell. "
.ε. is not force force but it is potential difference.
⇒ Consider resistor R connected to cell as shown in figure.
⇒ In resistor R, current I flows from C to D.
⇒ In electrolyte steady current flows from negative terminal (N) to positive tenninal (P) and in resistance current Hows from P to N.
⇒ If resistance R is infinite then,
I=VR=Vinfinite=0
where, V is potential difference between P and N.
∴V=[p.d. between p and N [ +
[ p.d. between A and B ] +
[ p.d. between B and N]
= V++(−Ir)+V−
=V++V−−Ir
ε=V++V−
In open circuit condition,
I = 0
∴V=ε
⇒ Thus, ε is potential difference between positive and negative electrode.
⇒ If R be finite resistance of the circuit then potential difference between P and N ,
V = ε−1r ...(2)
⇒ Practically If ε> Ir then internal resistance of cell can be neglected.
⇒ For different cell internal resistance is of different value .
⇒ Internal resistance of dry cell is large compared to internal resistance of electrolytic cell.
⇒ When current I flows through R, then p.d. between two end of resistance,
V = IR ... (3)
from equation (2) and (3)
IR = ε- Ir
or I = εr+R
when R = 0 maximum current tlow through cell.
∴Imax=εR
Remember :
(1) When cell is not in use then,
V = ε
(2) In case of open circuit condition,
V=ε
(3) When cell is in use , (V<ε)
(4) When cell is being charged, (V>ε)
∴V=ε+Ir
Similar Questions
Write down the relation between the emf of cell and its internal resistance.
View SolutionFind relation between emf and internal resistance of a cell.
View SolutionA cell of emf E is connected across a resistance R. The potential difference between the terminals of the cell is found to be V. The internal resistance of the cell must be -
View SolutionWhat are the differences between terminal potential difference and emf of the cell. Deduce an expression for internal resistance of a cell.
View SolutionA cell of emf E and internal resistance r is connected in series with an external resistance nr. Then, the ratio of the terminal potential difference to emf is :
View SolutionWhat are the differences between terminal potential difference and emf of the cell. Deduce an expression for internal resistance of a cell.
View SolutionThe internal resistance of a cell is the resistance of
View SolutionWhat is e.m.f. of a cell ? On what factors does its value depend ?
View Solution
