Which of the following reaction(s) can be used for the preparation of alkyl halides ?
(I) CH3CH2OH+HClanh.ZnCl2−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClanh.ZnCl2−−−−−→
(IV) only
(III) and (IV) only
(I), (II) and (IV) only
(I) and (II) only
The correct Answer is:C
(I) and (IV) can be used due to presence of anhydrous ZnCl2 (III) gives alkyl halide due to formation of more stable carbocation.
Topper's Solved these Questions
- ALCOHOLS , PHENOLS AND ETHERSBOOK - KUMAR PRAKASHANCHAPTER - ALCOHOLS , PHENOLS AND ETHERSEXERCISE - SECTION-E (MCQs asked in Board Exams)
63 Videos
- ALCOHOLS , PHENOLS AND ETHERSBOOK - KUMAR PRAKASHANCHAPTER - ALCOHOLS , PHENOLS AND ETHERSEXERCISE - SECTION-E (MCQs asked in Competitive Exam)
86 Videos
- BIOMOLECULESBOOK - KUMAR PRAKASHANCHAPTER - BIOMOLECULESEXERCISE - SECTION - E (MCQs asked in Board Exams)
61 Videos
Similar Questions
By which process phenyl acetate can be converted in to hydroxy acetophenone ?
View SolutionWhich product obtained when diethyl ether is heated with Conc. HI ?
View SolutionWhich solvent is used for cellulose ?
View SolutionExplain and complete the following reactions :
CH3−CH2−CH3∣C∣CH3−CH2OHHCl/ZnCl2−−−−−−−−−→Δ (b)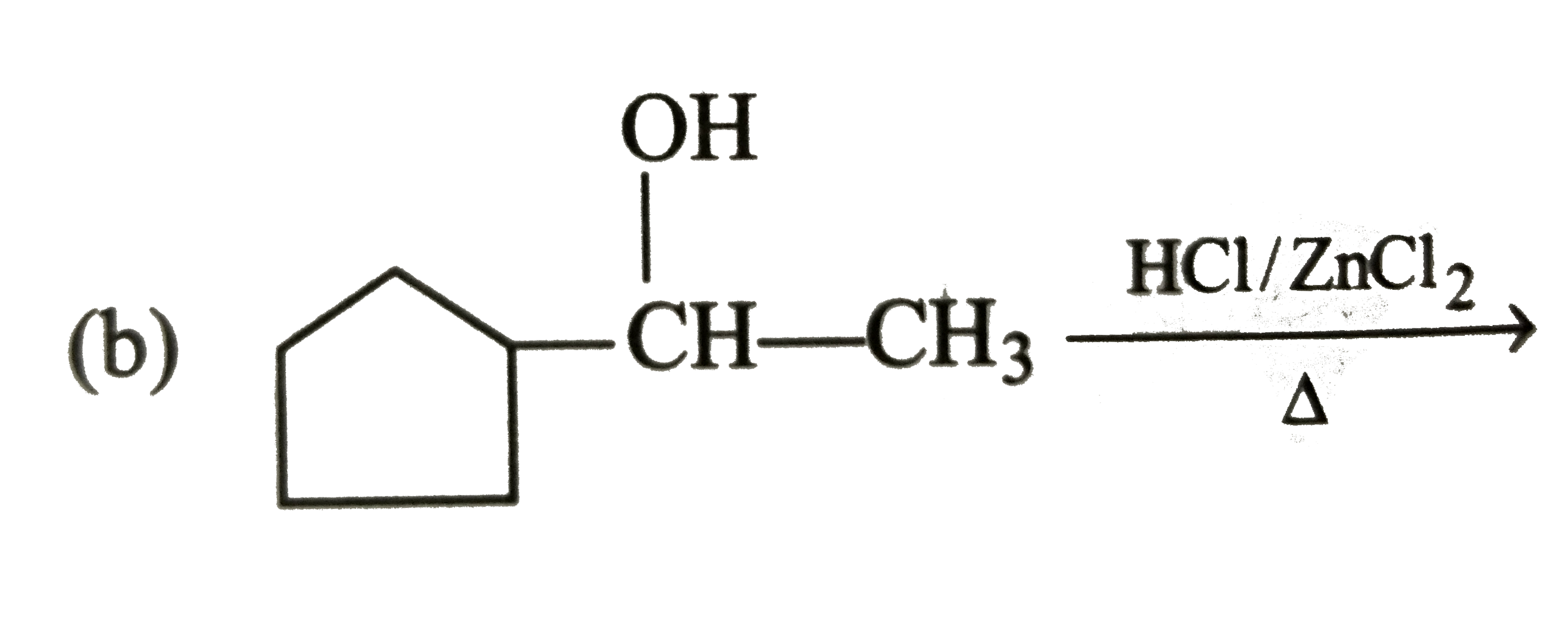
View SolutionAlcohols (i) CH3CH2CH2OH,(ii)CH3−CHOH−CH3 and (iii)CH3−C(CH3)(OH)−CH3 were treated with Lucas reagent (Conc. HCl+ZnCl2). What results do you expect at room temperature
View SolutionWhich of the following reaction(s) can be used for the preparation of alkyl halides ?
(I) CH3CH2OH+HClanhydrous ZnCl2−−−−−−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClanhydrousZnCl2−−−−−−−−−→
View SolutionWhich of the following reaction(s) can be used for the preparation of alkyl halides? (I) CH_(3)CH_(2)OH+HCl overset("anhyd. "ZnCl_(2))to (II) CH_(3)CH_(2)OH+HCl to (III) (CH_(3))_(3)COH+HCl to (IV) (CH_(3))_(2)CHOH+HCl overset(ZnCl_(2))to
View SolutionAssertion (A): (CH3)2−C∣CH3−OH+HClanhy−−−→ZnCl2
No reaction at Room temperature
Reason(R): (CH3)2−C∣CH3−OH+HCl is a secondary alcohol.
View SolutionWhich of the following compounds gives fastest reaction with HBr and why?
(i) (CH3)3_OH (ii) CH3CH2CH2OH
(iii) CH3OH∣CHCH3 (iv) CH3CH3∣CHCH2OH
View Solutionनिम्नलिखित में से कौनसी अभिक्रिया / अभिक्रियाएँ ऐल्किल हैलाइड के विरचन में उपयोग में ला सकते हैं
(I) CH3CH2OH+HClनिर्जलZnCl2−−−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClनिर्जलZnCl2−−−−−−−→
View SolutionWhich of the following reaction(s) can be used for the preparation of alkyl halides?
(I) CH3CH2OH+HClanhyd.ZnCl2−−−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClanhyd.ZnCl2−−−−−−−→
View SolutionWhich of the following reaction(s) can be used for the preparation of alkyl halides? (I) CH_(3)CH_(2)OH+HCl overset("anhyd." ZnCl_(2))to (II) CH_(3)CH_(2) OH+HCl to (III) (CH_(3))_(3)COH+HCl to (IV) (CH_(3))_(2)CHOH+HCl overset("anhyd." ZnCl_(2))to
View SolutionCH3CH2OH+HClZnCl2−−−→CH3CH2Cl+H2O
In the above reaction, the leaving group is
View SolutionWhich of the following reaction(s) can be used for the preparation of alkyl halides?
(I) CH3CH2OH+HClanhy.ZnCl2−−−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClanhy.ZnCl2−−−−−−−→
View Solutionनिम्नलिखित में से कौनसी अभिक्रिया / अभिक्रियाएँ ऐल्किल हैलाइड के विरचन में उपयोग में ला सकते हैं
(I) CH3CH2OH+HClनिर्जलZnCl2−−−−−−−→
(II) CH3CH2OH+HCl→
(III) (CH3)3COH+HCl→
(IV) (CH3)2CHOH+HClनिर्जलZnCl2−−−−−−−→
View Solution

