Which of the following is true for a spontaneous process?Solution in Telugu
There are one or more planes of symmetry
A single molecule is identical to its mirror image
More than one stereogenic centre must be present
The stereochemical lables, (R) and (S), must be identical for each sterogenic center
The correct Answer is:A, B, C
a) There are one or more planes of symmetry
b) A single molecule is identical to its mirror image
c) More than one stereogenic centre must be present
Topper's Solved these Questions
- ISOMERISM BOOK - AAKASH SERIESCHAPTER - ISOMERISM EXERCISE - PRACTICE SHEET EXERCISE-III (Stereo Isomerism) LEVEL-1I (ADVANCED) Linked Comprehension Type Questions (Passage - I)
3 Videos
- ISOMERISM BOOK - AAKASH SERIESCHAPTER - ISOMERISM EXERCISE - PRACTICE SHEET EXERCISE-III (Stereo Isomerism) LEVEL-1I (ADVANCED) Matrix Matching Type Questions
1 Video
- ISOMERISM BOOK - AAKASH SERIESCHAPTER - ISOMERISM EXERCISE - PRACTICE SHEET EXERCISE-III (Stereo Isomerism) LEVEL-1I (ADVANCED) Straight Objective Type Questions
26 Videos
- IONIC EQUILIBRIUM BOOK - AAKASH SERIESCHAPTER - IONIC EQUILIBRIUM EXERCISE - ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))
8 Videos
- PERIODIC CLASSIFICATIONBOOK - AAKASH SERIESCHAPTER - PERIODIC CLASSIFICATIONEXERCISE - OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)
14 Videos
Similar Questions
The necessary condition for a molecule to exhibit optical isomerism is dissymmetry or chiral. Thus all organic compounds which contain one assymetric carbon atom are chiral and exist in two stereoisomers. Although the two forms have the same structure, they have different arrangements of groups about the assymmetric carbon. In fact, they represent assymetric molecules. They do not have a plane of symmetry. They are related to each other as an object to its miror image and are non superimposable. The two structures actually stand for dextro or (+) and leavo or (-) isomers. Since they are related each other as mirror images, they are commonly called enantiomers.
Consider the following structers A,B,C,D
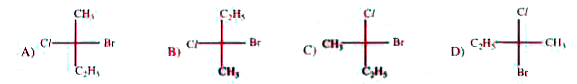
Which of the following statements is not correctConsider the following structers A,B,C,D

Which of the following statements is not correct
View SolutionMatch the following columns
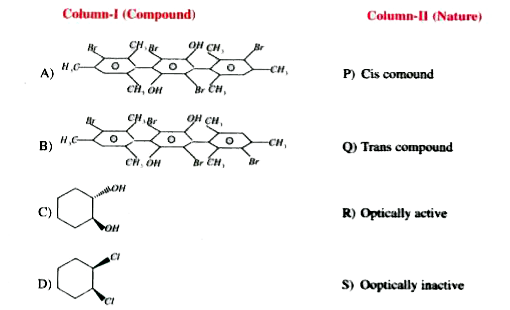
View SolutionMaximum number of possible stereoisomers (configurational only) with the formula CH3CH=CHCH(CH3)COOH
View SolutionA certain weak acid has Ka,=10−5. If the equilibrium constant for is its reaction with a strong base is repesented as y×10y then find the value of y.
View SolutionWhich one of the following orders is not in accordance with the property stated against it?
View SolutionFor a spontaneous process, which of the following is always true?
View SolutionWhich of the following is correct for a spontaneous process?
View SolutionFor a spontaneous process, which of the following is true?
View SolutionWhich of the following Process is non-spontaneous
View SolutionWhich of the following is true for spontaneous process?
View SolutionWhich of the following processes is a non-spontaneous process?
View SolutionWhich of the following is non-spontaneous process?
View SolutionWhich one of the following is true for a spontaneous process-
View SolutionWhich of the following is not a spontaneous process?
View SolutionFor a process to be spontaneous at all conditions of temperature which of the following is true?
View SolutionWhich of the following is always true for a spontaneous process
View Solution

