Which of the following statements best explain (s) the relationship between two stereoisomers of CH3CH=CHCH3 ?Solution in Telugu
They are diastereomers
They are enantiomers
They are optical isomers
Their melting points are identical
The correct Answer is:A
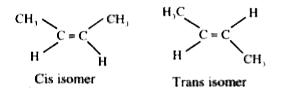
Cis isomer Trans isomer
Cis and Trans isomers ⇒ diastereomers
Similar Questions
Arrange the following in the increasing order of stability. CH_(3)CH_(2)CH=CH_(2), CH_(3)CH=CHCH_(3)(cis), CH_(3)CH=CHCH_(3)" (trans)"
View SolutionArrange the following in the increasing order of stability.
CH3CH2CH=CH2,CH3CH=CHCH3(cis),CH3CH=CHCH3 (trans)
View SolutionArrange the following in the increasing order of stability.
CH3CH2CH=CH2,CH3CH=CHCH3(cis),CH3CH=CHCH3 (trans)
View SolutionArrange the following in the increasing order of stability.
CH3CH2CH=CH2,CH3CH=CHCH3(cis),CH3CH=CHCH3 (trans)
View SolutionWhich of the following compounds exhibits geometrical isomerism? underset((i))(CH_(2)=CHCH_(3)) underset((ii))(CH_(3)CH=CHCH_(3)) underset((iii))(CH_(3)CH=C)=CHCH_(3) underset((iv))(CH_(3)CH=C=C=CHCH_(3))
View SolutionStatement (S) : Cis and trans geometrical isomers of CH3CH=CHCH3 is possible.
Reason (R) : Free radical rotation surrounding carbon-carbon double bond is not possible in CH3CH=CHCH3.
View SolutionTotal number of optical active stereoisomers of the following compound is :
CH3−CH=CH−CHOH−CH=CH−CH3
View Solution
