From the following dipole moment (in Debye) values of methyl halides, identify the value of CH3−FSolution in Telugu
1.460
1.636
1.730
1.847
The correct Answer is:C
Step by step video, text & image solution for From the following dipole moment (in Debye) values of methyl halides, identify the value of CH_(3)-F by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Among the following methyl halides the one with the highest value of dipole moment is :
View SolutionThe dipole moment of
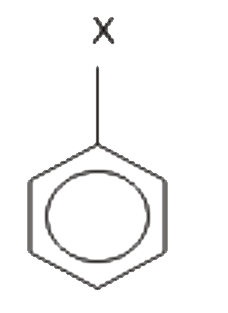 is 1.5 D.
is 1.5 D.
The dipole Moment of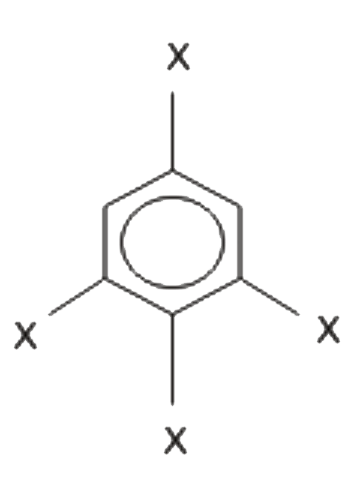 in Debye
in Debye
View SolutionCH3F का द्विध्रुव आघूर्ण CH3Cl से अधिक है।
View Solutionयदि HCl अणु पूरी तरह धुवीय है तो द्विध्रुव आघूर्ण का आपेक्षित मान 6.12D (debye) है, लेकिन (द्विध्रुव आघूर्ण का) प्रायोगिक मान 1.03D है। प्रतिशत आयनिक गुण की गणना करो
View SolutionThe dipole moments of CH3F,CH3ClandCH3Br are in the order :
View SolutionDebye an unit of dipole moment is of the order of :
View SolutionAmong the following methyl halides the one with the highest value of dipole moment is :
View Solution

