Combustion of 0.277g of an organic compound gave 0.66g carbondioxide and 0.337g water. Vapour density of the compound is equal to 37. Calculate its molecular formula. Solution in Telugu
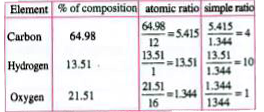
% of Carbon =1244×0.660.277×100=64.98
% of Hydrogen =218×0.3370.277×100=13.51
% of Oxygen =100−(64.98+13.51)=21.51
Empirical formula of the compound =C4H10O
Empirical formula weight =(4×12)+(10×1)+(1×16)=74
n=Molecular weightEmpirical formula weight=2×3774=1
Molecular formula =(Empirical formula)×n
=(C4H10O)1=C4H10O
Similar Questions
Combustion of 0.6gm of an organic compound gave 1.17gm of carbondioxide. 0.84 gm of water. Vapour density of the compound is equal to 22.4. The compound contains carbon, hydrogen and nitrogen. Calculate the molecular formula.
View Solution0.2614g of an organic compound gave upon combustion 0.2324g of CO_(2) and 0.0950g of H_(2)O . 0.1195g of this compound gave 0.3470g of AgCl. If the vapour density of the substance is 49.5, calculate its molecular formula.
View SolutionAn organic compound has an empirical formula CH2O. Its vapour density is 45. The molecular formula of the compound is
View SolutionA compound with empirical formula XY2 has vapour density equal to its empirical formula weight its molecular formula is
View Solution0.246g of an organic compound on complete combustion gave 0.198g of Carbondioxide and 0.1014g of water,then the percentage composition of hydrogen in the compound is
View SolutionAn organic compound contains 49.3% carbon, 6.84% hydrogen
and 43.86% of oxygen and its vapour density is 73 . Molecular formula of the compound is
View SolutionOn complete combustion of 0.246g of an organic compound gave 0.198g of carbondioxide and 0.1014g of water. Determine the percentage combustion of carbon and hydrogen in the compound.
View Solution
